Inhaled Nitric Oxide Market Analysis and Global Forecast 2023-2033
$ 1,390.00 – $ 5,520.00Price range: $ 1,390.00 through $ 5,520.00
Inhaled Nitric Oxide Market Research Report: Information By Product Type (Neonatal Respiratory Treatment, Chronic Obstructive Pulmonary Diseases, Acute Respiratory Distress Syndrome, Others), By Application (Delivery Systems, Gas), and by Region — Forecast till 2033
Page: 161
Inhaled Nitric Oxide Market Overview
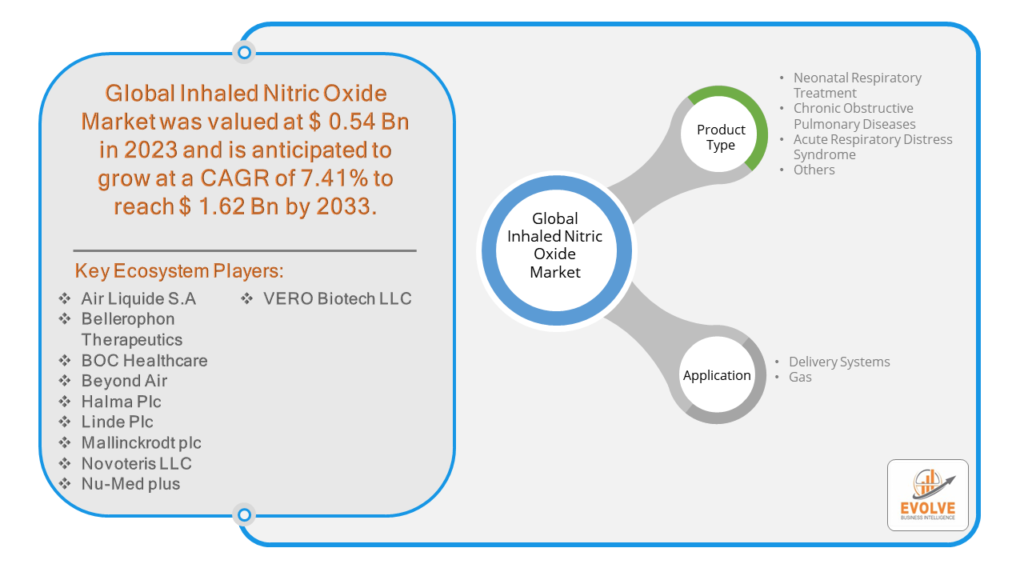
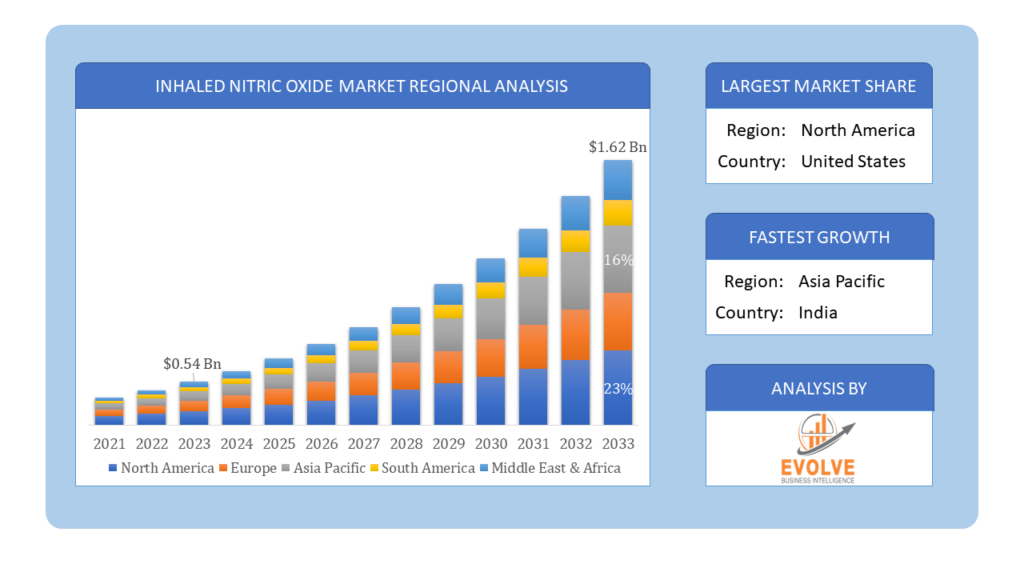
The Inhaled Nitric Oxide Market Size is expected to reach USD 1.62 Billion by 2033. The Inhaled Nitric Oxide Market industry size accounted for USD 0.54 Billion in 2023 and is expected to expand at a compound annual growth rate (CAGR) of 7.41% from 2023 to 2033. The Inhaled Nitric Oxide Market refers to the commercial landscape surrounding the production, distribution, and usage of inhaled nitric oxide (iNO) as a medical treatment. Inhaled nitric oxide is a vasodilator used primarily in the treatment of respiratory conditions and complications.
The inhaled nitric oxide market is characterized by its role in critical care and respiratory treatment, its technological advancements, and the regulatory environment that governs its usage.
Global Inhaled Nitric Oxide Market Synopsis
The COVID-19 pandemic has significantly impacted the Inhaled Nitric Oxide (iNO) market. There was a surge in the demand for inhaled nitric oxide as a potential treatment for COVID-19, especially for severe respiratory complications. iNO’s properties as a vasodilator made it a candidate for improving oxygenation in patients with severe respiratory distress. Numerous clinical trials were initiated to investigate the efficacy of iNO in treating COVID-19, leading to a heightened focus on its potential benefits and safety. The pandemic caused disruptions in the manufacturing and supply chain of medical gases and related equipment. Lockdowns and restrictions impacted production facilities and logistics. The pandemic has spurred ongoing research into the broader applications of inhaled nitric oxide beyond COVID-19, potentially expanding its use in other respiratory conditions. Improvements in manufacturing and delivery infrastructure for iNO are likely to have long-term benefits, ensuring better preparedness for future health crises.
Inhaled Nitric Oxide Market Dynamics
The major factors that have impacted the growth of Inhaled Nitric Oxide Market are as follows:
Drivers:
Ø Technological Advancements
Innovations in inhaled nitric oxide delivery systems, including portable and integrated devices, have made treatment more efficient and accessible. Modern delivery systems incorporate safety features that ensure precise dosing and minimize risks, boosting market adoption. Growing awareness among healthcare providers about the benefits of iNO therapy leads to higher prescription rates. Enhanced patient awareness and advocacy for effective treatments contribute to increased demand. The improvement of healthcare infrastructure in emerging markets provides new opportunities for market expansion. Increased healthcare spending in developing countries supports the adoption of advanced respiratory treatments, including iNO.
Restraint:
- Perception of High Cost of Treatment
The high cost associated with inhaled nitric oxide therapy and the delivery systems can limit its accessibility and affordability, particularly in low- and middle-income countries.Limited insurance coverage and reimbursement policies for iNO therapy can deter healthcare providers and patients from opting for this treatment. The requirement for continuous monitoring and specialized equipment to ensure safe administration of iNO can be a barrier, especially in resource-limited settings. The presence of alternative therapies and treatments for respiratory conditions, such as other vasodilators, mechanical ventilation, and ECMO (extracorporeal membrane oxygenation), can limit the adoption of iNO.
Opportunity:
⮚ Expansion of Therapeutic Indications
Ongoing research into the use of iNO for various respiratory and non-respiratory conditions can open new therapeutic areas, such as chronic obstructive pulmonary disease (COPD), asthma, and cardiovascular diseases. Development of more efficient, user-friendly, and portable iNO delivery systems can enhance patient compliance and expand usage in outpatient and homecare settings. Continued focus on the use of iNO in neonatal care, particularly for conditions like hypoxic respiratory failure and pulmonary hypertension, can drive demand. Expanding research and clinical trials to explore additional pediatric applications can create new market opportunities.
Inhaled Nitric Oxide Market Segment Overview
By Product Type
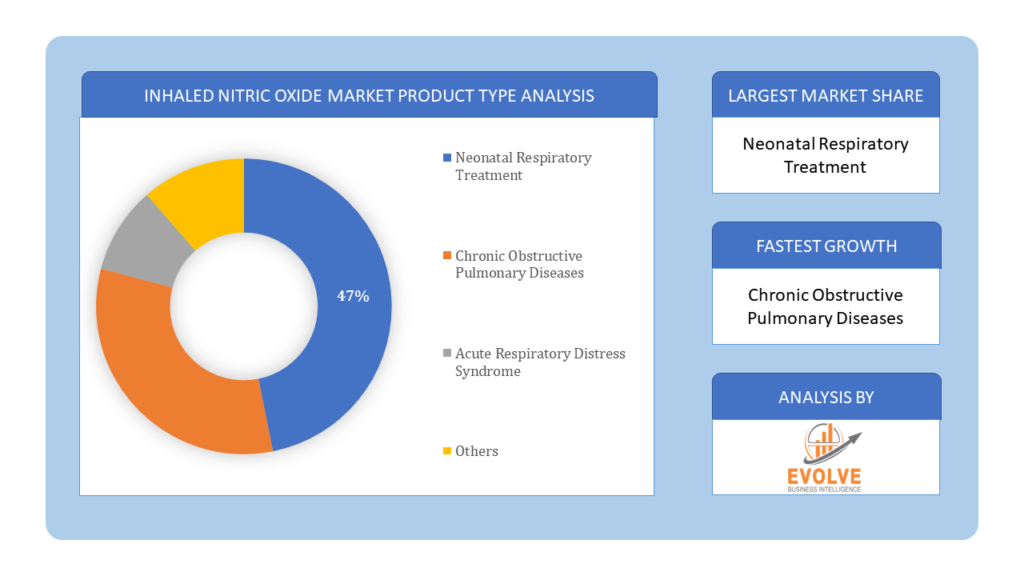 Based on Product Type, the market is segmented based on Neonatal Respiratory Treatment, Chronic Obstructive Pulmonary Diseases, Acute Respiratory Distress Syndrome and Others. Neonatal respiratory treatment is the dominating segment in the inhaled nitric oxide market. It is used to treat newborn infants with persistent pulmonary hypertension. It helps to improve oxygenation and reduce the need for extracorporeal membrane oxygenation in neonates. Nitric oxide is generated endogenously from L-arginine by nitric oxide synthase. Nitric oxide diffuses into vascular smooth muscle cells processing towards vasodilation and enhanced ventilation-perfusion matching.
Based on Product Type, the market is segmented based on Neonatal Respiratory Treatment, Chronic Obstructive Pulmonary Diseases, Acute Respiratory Distress Syndrome and Others. Neonatal respiratory treatment is the dominating segment in the inhaled nitric oxide market. It is used to treat newborn infants with persistent pulmonary hypertension. It helps to improve oxygenation and reduce the need for extracorporeal membrane oxygenation in neonates. Nitric oxide is generated endogenously from L-arginine by nitric oxide synthase. Nitric oxide diffuses into vascular smooth muscle cells processing towards vasodilation and enhanced ventilation-perfusion matching.
By Application
Based on Application, the market segment has been divided into the Delivery Systems and Gas. Gas is the dominating segment in the inhaled nitric oxide market. Persistent Pulmonary Hypertension of the Newborn is a condition where the normal circulatory transition after birth fails to occur, resulting in high pulmonary artery pressure and poor oxygenation. Inhaled nitric oxide dilates the pulmonary vessels, improving blood flow and oxygenation.
Global Inhaled Nitric Oxide Market Regional Analysis
Based on region, the global Inhaled Nitric Oxide Market has been divided into North America, Europe, Asia-Pacific, the Middle East & Africa, and Latin America. North America is projected to dominate the use of the Inhaled Nitric Oxide Market followed by the Asia-Pacific and Europe regions.
 Inhaled Nitric Oxide North America Market
Inhaled Nitric Oxide North America Market
North America holds a dominant position in the Inhaled Nitric Oxide Market. North America, particularly the United States, holds a significant share of the global iNO market. This is driven by advanced healthcare infrastructure, high healthcare expenditure, and a strong presence of key market players. The FDA’s approvals and guidelines play a crucial role in the market. The region benefits from a robust regulatory framework that supports the development and adoption of new iNO therapies. High investment in R&D and numerous clinical trials focusing on the efficacy of iNO for various indications contribute to market growth.
Inhaled Nitric Oxide Asia-Pacific Market
The Asia-Pacific region has indeed emerged as the fastest-growing market for the Inhaled Nitric Oxide Market industry. The Asia-Pacific region is experiencing rapid market growth due to increasing healthcare investments, improving healthcare infrastructure, and rising awareness about advanced respiratory therapies. The regulatory landscape varies significantly across the region, with some countries having more streamlined processes than others. Efforts to penetrate rural and underserved areas, combined with government initiatives to improve healthcare access, are driving market expansion.
Competitive Landscape
The global Inhaled Nitric Oxide Market is highly competitive, with numerous players offering a wide range of software solutions. The competitive landscape is characterized by the presence of established companies, as well as emerging startups and niche players. To increase their market position and attract a wide consumer base, the businesses are employing various strategies, such as product launches, and strategic alliances.
Prominent Players:
- Air Liquide S.A
- Bellerophon Therapeutics
- BOC Healthcare
- Beyond Air
- Halma Plc
- Linde Plc
- Mallinckrodt plc
- Novoteris LLC
- Nu-Med plus
- VERO Biotech LLC
Key Development
In February 2023, Vero Biotech declared that the FDA accepted Genosyl, second-generation, for rebreathing anesthesia in the functional room setting. Vero Biotech made Genosyl for the delivery of inhaled nitric oxide, which underlines the second-generation Genosyl. The third-generation device recently obtained FDA approval has yet to be verified with rebreathing anesthesia.
Scope of the Report
Global Inhaled Nitric Oxide Market, by Product Type
- Neonatal Respiratory Treatment
- Chronic Obstructive Pulmonary Diseases
- Acute Respiratory Distress Syndrome
- Others
Global Inhaled Nitric Oxide Market, by Application
- Delivery Systems
- Gas
Global Inhaled Nitric Oxide Market, by Region
- North America
- US
- Canada
- Mexico
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Benelux
- Nordic
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- Indonesia
- Austalia
- Malaysia
- India
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- South Africa
- Rest of Middle East & Africa
| Parameters | Indicators |
|---|---|
| Market Size | 2033: $1.62 Billion |
| CAGR | 7.41% CAGR (2023-2033) |
| Base year | 2022 |
| Forecast Period | 2023-2033 |
| Historical Data | 2021 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, and Trends |
| Key Segmentations | Product Type, Application |
| Geographies Covered | North America, Europe, Asia-Pacific, Latin America, Middle East, Africa |
| Key Vendors | Air Liquide S.A, Bellerophon Therapeutics, BOC Healthcare, Beyond Air, Halma Plc, Linde Plc, Mallinckrodt plc, Novoteris LLC, Nu-Med plus and VERO Biotech LLC |
| Key Market Opportunities | • Expansion of Therapeutic Indications • Focus on Pediatric and Neonatal Care |
| Key Market Drivers | • Technological Advancements • Increasing Awareness and Adoption |
REPORT CONTENT BRIEF:
- High-level analysis of the current and future Inhaled Nitric Oxide Market trends and opportunities
- Detailed analysis of current market drivers, restraining factors, and opportunities in the future
- Inhaled Nitric Oxide Market historical market size for the year 2021, and forecast from 2023 to 2033
- Inhaled Nitric Oxide Market share analysis at each product level
- Competitor analysis with detailed insight into its product segment, Government & Defense strength, and strategies adopted.
- Identifies key strategies adopted including product launches and developments, mergers and acquisitions, joint ventures, collaborations, and partnerships as well as funding taken and investment done, among others.
- To identify and understand the various factors involved in the global Inhaled Nitric Oxide Market affected by the pandemic
- To provide a detailed insight into the major companies operating in the market. The profiling will include the Government & Defense health of the company’s past 2-3 years with segmental and regional revenue breakup, product offering, recent developments, SWOT analysis, and key strategies.
Press Release

Global Pharmaceutical Manufacturing Market to Reach $1.38 Trillion by 2035 with 7.35% CAGR, New Research Shows

The Global Mammography Market Is Estimated To Record a CAGR of Around 10.29% During The Forecast Period

Glue Stick Market to Reach USD 2.35 Billion by 2034

Podiatry Service Market to Reach USD 11.88 Billion by 2034

Microfluidics Technology Market to Reach USD 32.58 Billion by 2034

Ferric Chloride Market to Reach USD 10.65 Billion by 2034

Family Practice EMR Software Market to Reach USD 21.52 Billion by 2034

Electric Hairbrush Market to Reach USD 15.95 Billion by 2034

Daily Bamboo Products Market to Reach USD 143.52 Billion by 2034

Cross-border E-commerce Logistics Market to Reach USD 112.65 Billion by 2034
Frequently Asked Questions (FAQ)
What is the study period of the Inhaled Nitric Oxide Market?
The study period for the Inhaled Nitric Oxide Market is from 2023 to 2033.
What is the growth rate of the Inhaled Nitric Oxide Market?
The Inhaled Nitric Oxide Market is expected to grow at a compound annual growth rate (CAGR) of 7.41% from 2023 to 2033.
Which region has the highest growth rate in the Inhaled Nitric Oxide Market?
The Asia-Pacific region has the highest growth rate in the Inhaled Nitric Oxide Market.
Which region has the largest share of the Inhaled Nitric Oxide Market?
North America holds the largest share of the Inhaled Nitric Oxide Market.
Who are the key players in the Inhaled Nitric Oxide Market?
Key players in the Inhaled Nitric Oxide Market include Air Liquide S.A, Bellerophon Therapeutics, BOC Healthcare, Beyond Air, Halma Plc, and Linde Plc.
Do you offer Post sales support?
Yes, we offer 16 hours of analyst support to solve the queries
Do you sell particular sections of a report?
Yes, we provide regional as well as country-level reports. Other than this we also provide a sectional report. Please get in contact with our sales representatives.
Table of Content
Chapter 1. Executive Summary Chapter 2. Scope Of The Study 2.1. Market Definition 2.2. Scope Of The Study 2.2.1. Objectives of Report 2.2.2. Limitations 2.3. Market Structure Chapter 3. Evolve BI Methodology Chapter 4. Market Insights and Trends 4.1. Supply/ Value Chain Analysis 4.1.1. Raw End Users Providers 4.1.2. Manufacturing Process 4.1.3. Distributors/Retailers 4.1.4. End-Use Industry 4.2. Porter’s Five Forces Analysis 4.2.1. Threat Of New Entrants 4.2.2. Bargaining Power Of Buyers 4.2.3. Bargaining Power Of Suppliers 4.2.4. Threat Of Substitutes 4.2.5. Industry Rivalry 4.3. Impact Of COVID-19 on the Inhaled Nitric Oxide Market 4.3.1. Impact on Market Size 4.3.2. End-Use Industry Trend, Preferences, and Budget Impact 4.3.3. Regulatory Framework/Government Policies 4.3.4. Key Players' Strategy to Tackle Negative Impact 4.3.5. Opportunity Window 4.4. Technology Overview 12.28. Macro factor 4.6. Micro Factor 4.7. Demand Supply Gap Analysis of the Inhaled Nitric Oxide Market 4.8. Import Analysis of the Inhaled Nitric Oxide Market 4.9. Export Analysis of the Inhaled Nitric Oxide Market Chapter 5. Market Dynamics 5.1. Introduction 5.2. DROC Analysis 5.2.1. Drivers 5.2.2. Restraints 5.2.3. Opportunities 5.2.4. Challenges 5.3. Patent Analysis 5.4. Industry Roadmap 5.5. Parent/Peer Market Analysis Chapter 6. Global Inhaled Nitric Oxide Market, By Product Type 6.1. Introduction 6.2. Neonatal Respiratory Treatment 6.3. Chronic Obstructive Pulmonary Diseases 6.4. Acute Respiratory Distress Syndrome 6.5. Others Chapter 7. Global Inhaled Nitric Oxide Market, By Application 7.1. Introduction 7.2. Delivery Systems 7.3. Gas Chapter 8. Global Inhaled Nitric Oxide Market, By Region 8.1. Introduction 8.2. North America 8.2.1. Introduction 8.2.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.2.3. Market Size and Forecast, By Country, 2023-2033 8.2.4. Market Size and Forecast, By Product Type, 2023-2033 8.2.5. Market Size and Forecast, By End User, 2023-2033 8.2.6. US 8.2.6.1. Introduction 8.2.6.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.2.6.3. Market Size and Forecast, By Product Type, 2023-2033 8.2.6.4. Market Size and Forecast, By End User, 2023-2033 8.2.7. Canada 8.2.7.1. Introduction 8.2.7.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.2.7.4. Market Size and Forecast, By Product Type, 2023-2033 8.2.7.5. Market Size and Forecast, By End User, 2023-2033 8.3. Europe 8.3.1. Introduction 8.3.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.3. Market Size and Forecast, By Country, 2023-2033 8.3.4. Market Size and Forecast, By Product Type, 2023-2033 8.3.5. Market Size and Forecast, By End User, 2023-2033 8.3.6. Germany 8.3.6.1. Introduction 8.3.6.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.6.3. Market Size and Forecast, By Product Type, 2023-2033 8.3.6.4. Market Size and Forecast, By End User, 2023-2033 8.3.7. France 8.3.7.1. Introduction 8.3.7.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.7.3. Market Size and Forecast, By Product Type, 2023-2033 8.3.7.4. Market Size and Forecast, By End User, 2023-2033 8.3.8. UK 8.3.8.1. Introduction 8.3.8.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.8.3. Market Size and Forecast, By Product Type, 2023-2033 8.3.8.4. Market Size and Forecast, By End User, 2023-2033 8.3.9. Italy 8.3.9.1. Introduction 8.3.9.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.9.3. Market Size and Forecast, By Product Type, 2023-2033 8.3.9.4. Market Size and Forecast, By End User, 2023-2033 8.3.11. Rest Of Europe 8.3.11.1. Introduction 8.3.11.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.11.3. Market Size and Forecast, By Product Type, 2023-2033 8.3.11.4. Market Size and Forecast, By End User, 2023-2033 8.4. Asia-Pacific 8.4.1. Introduction 8.4.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.3. Market Size and Forecast, By Country, 2023-2033 8.4.4. Market Size and Forecast, By Product Type, 2023-2033 8.12.28. Market Size and Forecast, By End User, 2023-2033 8.4.6. China 8.4.6.1. Introduction 8.4.6.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.6.3. Market Size and Forecast, By Product Type, 2023-2033 8.4.6.4. Market Size and Forecast, By End User, 2023-2033 8.4.7. India 8.4.7.1. Introduction 8.4.7.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.7.3. Market Size and Forecast, By Product Type, 2023-2033 8.4.7.4. Market Size and Forecast, By End User, 2023-2033 8.4.8. Japan 8.4.8.1. Introduction 8.4.8.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.8.3. Market Size and Forecast, By Product Type, 2023-2033 8.4.8.4. Market Size and Forecast, By End User, 2023-2033 8.4.9. South Korea 8.4.9.1. Introduction 8.4.9.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.9.3. Market Size and Forecast, By Product Type, 2023-2033 8.4.9.4. Market Size and Forecast, By End User, 2023-2033 8.4.10. Rest Of Asia-Pacific 8.4.10.1. Introduction 8.4.10.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.10.3. Market Size and Forecast, By Product Type, 2023-2033 8.4.10.4. Market Size and Forecast, By End User, 2023-2033 8.5. Rest Of The World (RoW) 8.5.1. Introduction 8.5.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.5.3. Market Size and Forecast, By Product Type, 2023-2033 8.5.4. Market Size and Forecast, By End User, 2023-2033 Chapter 9. Company Landscape 9.1. Introduction 9.2. Vendor Share Analysis 9.3. Key Development Analysis 9.4. Competitor Dashboard Chapter 10. Company Profiles 10.1. Air Liquide S.A 10.1.1. Business Overview 10.1.2. Government & Defense Analysis 10.1.2.1. Government & Defense – Existing/Funding 10.1.3. Product Portfolio 10.1.4. Recent Development and Strategies Adopted 10.1.5. SWOT Analysis 10.2. Bellerophon Therapeutics 10.2.1. Business Overview 10.2.2. Government & Defense Analysis 10.2.2.1. Government & Defense – Existing/Funding 10.2.3. Product Portfolio 10.2.4. Recent Development and Strategies Adopted 10.2.5. SWOT Analysis 10.3. BOC Healthcare 10.3.1. Business Overview 10.3.2. Government & Defense Analysis 10.3.2.1. Government & Defense – Existing/Funding 10.3.3. Product Portfolio 10.3.4. Recent Development and Strategies Adopted 10.3.5. SWOT Analysis 10.4. Beyond Air 10.4.1. Business Overview 10.4.2. Government & Defense Analysis 10.4.2.1. Government & Defense – Existing/Funding 10.4.3. Product Portfolio 10.4.4. Recent Development and Strategies Adopted 10.12.28. SWOT Analysis 10.5. Halma Plc 10.5.1. Business Overview 10.5.2. Government & Defense Analysis 10.5.2.1. Government & Defense – Existing/Funding 10.5.3. Product Portfolio 10.5.4. Recent Development and Strategies Adopted 10.5.5. SWOT Analysis 10.6. Linde Plc 10.6.1. Business Overview 10.6.2. Government & Defense Analysis 10.6.2.1. Government & Defense – Existing/Funding 10.6.3. Product Portfolio 10.6.4. Recent Development and Strategies Adopted 10.6.5. SWOT Analysis 10.7. Mallinckrodt plc 10.7.1. Business Overview 10.7.2. Government & Defense Analysis 10.7.2.1. Government & Defense – Existing/Funding 10.7.3. Product Portfolio 10.7.4. Recent Development and Strategies Adopted 10.7.5. SWOT Analysis 10.8 Novoteris LLC 10.8.1. Business Overview 10.8.2. Government & Defense Analysis 10.8.2.1. Government & Defense – Existing/Funding 10.8.3. Product Portfolio 10.8.4. Recent Development and Strategies Adopted 10.8.5. SWOT Analysis 10.9 Nu-Med plus 10.9.1. Business Overview 10.9.2. Government & Defense Analysis 10.9.2.1. Government & Defense – Existing/Funding 10.9.3. Product Portfolio 10.9.4. Recent Development and Strategies Adopted 10.9.5. SWOT Analysis 10.10. VERO Biotech LLC 10.10.1. Business Overview 10.10.2. Government & Defense Analysis 10.10.2.1. Government & Defense – Existing/Funding 10.10.3. Product Portfolio 10.10.4. Recent Development and Strategies Adopted 10.10.5. SWOT Analysis
Connect to Analyst
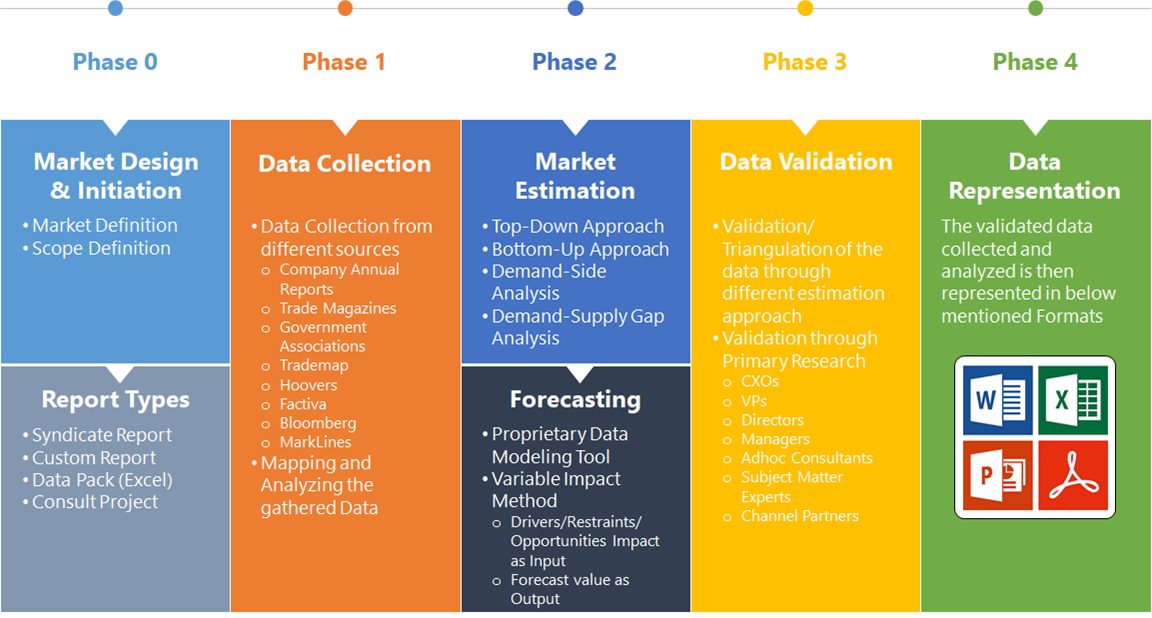
Research Methodology