Cardiac Resynchronization Therapy Market Analysis and Global Forecast 2023-2033
€ 3,071.64 – € 10,082.43Price range: € 3,071.64 through € 10,082.43
Cardiac Resynchronization Therapy Market Research Report: Information Product Type (Cardiac Resynchronization Therapy Defibrillators, Cardiac Resynchronization Therapy Pacemakers), By End User (Hospitals & Cardiac Centers, Ambulatory Surgery Centers), and by Region — Forecast till 2033
Page: 171
Cardiac Resynchronization Therapy Market Overview
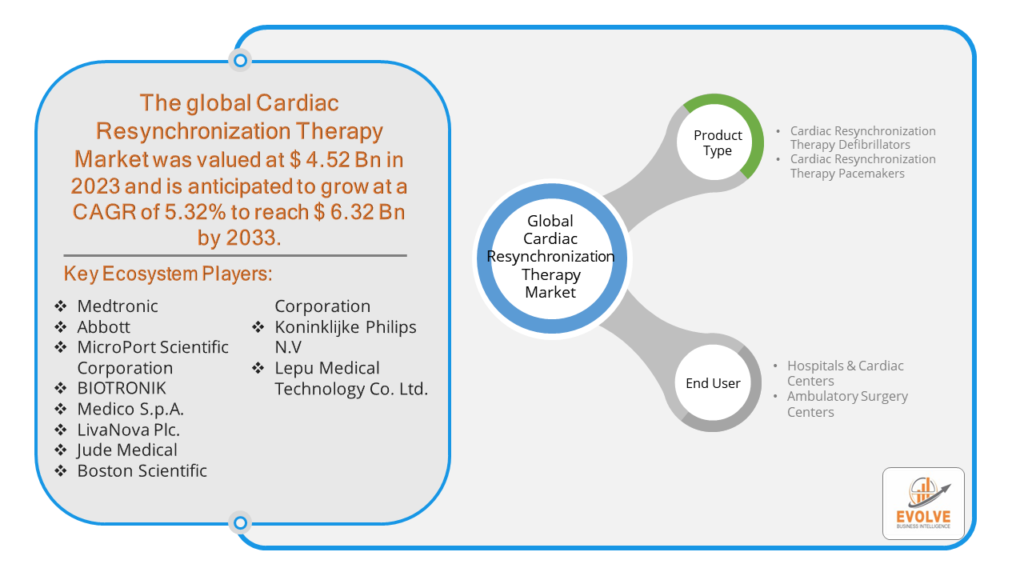
The Cardiac Resynchronization Therapy Market Size is expected to reach USD 6.32 Billion by 2033. The Cardiac Resynchronization Therapy Market industry size accounted for USD 4.52 Billion in 2023 and is expected to expand at a compound annual growth rate (CAGR) of 5.32% from 2023 to 2033. Cardiac Resynchronization Therapy (CRT) is a medical treatment aimed at improving the heart’s function in patients with heart failure by coordinating the contractions of the heart’s ventricles. This is achieved through the implantation of a specialized pacemaker device that sends electrical impulses to both the left and right ventricles to help them contract in sync. By enhancing the timing of these contractions, CRT improves the heart’s efficiency in pumping blood, alleviating symptoms of heart failure, enhancing exercise capacity, and reducing the risk of hospitalization and mortality associated with the condition. CRT is particularly beneficial for patients with severe heart failure and specific electrical conduction abnormalities, such as left bundle branch block.
The COVID-19 pandemic had a mixed impact on the Cardiac Resynchronization Therapy (CRT) market. Elective procedures, including CRT device implants, were often delayed or canceled due to hospital capacity being redirected to handle COVID-19 cases, leading to a temporary decline in new implants. However, the pandemic also heightened awareness about managing chronic conditions like heart failure, potentially increasing future demand for CRT. Furthermore, the crisis accelerated the adoption of telemedicine and remote monitoring technologies, enabling continued care for patients with CRT devices despite restrictions on in-person visits, thus driving innovation and adaptation within the market.
Global Cardiac Resynchronization Therapy Market Synopsis
The COVID-19 pandemic had a mixed impact on the Cardiac Resynchronization Therapy Market. Initially, the market experienced disruptions due to lockdown measures, supply chain interruptions, and reduced construction activity in many parts of the world. This led to a temporary decline in demand for Cardiac Resynchronization Therapy and related construction materials. As the construction sector gradually resumed operations and governments implemented stimulus packages to support infrastructure development, the demand for Cardiac Resynchronization Therapy began to recover. Additionally, the shift towards remote work and increased time spent at home during the pandemic boosted renovation and remodeling activities, driving demand for gypsum-based products such as drywall. The pandemic highlighted the importance of sustainable building practices and materials, including Cardiac Resynchronization Therapy, which is seen as an environmentally friendly alternative to natural gypsum. This increased awareness of sustainability issues could further drive the adoption of Cardiac Resynchronization Therapy in construction projects post-pandemic.
Cardiac Resynchronization Therapy Market Dynamics
The major factors that have impacted the growth of Cardiac Resynchronization Therapy Market are as follows:
Drivers:
Ø Rising Prevalence of Cardiovascular Diseases
The rising prevalence of cardiovascular diseases (CVDs), particularly heart failure and arrhythmias, is a major driver for the cardiac resynchronization therapy (CRT) market. With an aging global population, the incidence of heart-related conditions continues to climb. Lifestyle factors such as poor diet, lack of exercise, smoking, and increased stress levels contribute significantly to the growing number of cardiovascular cases. As these conditions often lead to heart failure and other severe complications, there is an increasing need for effective treatment options. CRT devices, which help coordinate the contractions of the heart’s ventricles, improve heart function and quality of life for patients suffering from heart failure. This growing patient population, coupled with increasing awareness and diagnosis of CVDs, is driving the demand for CRT devices and therapies.
Restraint:
- High Cost and Reimbursement Issues
The high cost of cardiac resynchronization therapy devices and procedures poses a significant restraint on the market. CRT devices, including implantation and follow-up care, represent a substantial financial burden for many patients and healthcare systems. These costs can be prohibitively high, particularly in low- and middle-income countries where healthcare funding is limited. Furthermore, reimbursement issues add another layer of complexity. Variability in coverage by insurance providers and stringent reimbursement policies can limit patient access to CRT. Patients often face out-of-pocket expenses, which can deter them from opting for this therapy. These financial barriers can slow down the adoption of CRT devices, restricting market growth, especially in regions with less developed healthcare infrastructures.
Opportunity:
⮚ Technological Advancements and Innovation
Technological advancements and ongoing innovations in CRT devices present significant opportunities for market growth. The development of miniaturized devices enhances patient comfort and reduces the risk of complications during and after implantation. Improvements in battery life extend the functionality and reduce the need for frequent replacements, thus lowering long-term costs and improving patient convenience. Enhanced device programming and remote monitoring capabilities allow for more precise and individualized patient care. Innovations such as leadless CRT devices and the integration of advanced imaging techniques for better implantation accuracy further improve clinical outcomes. Additionally, the growing use of telemedicine and remote patient monitoring systems enables continuous monitoring of device performance and patient condition, leading to timely interventions and better management of heart failure symptoms. These technological advancements not only improve patient outcomes but also expand the potential market for CRT devices by making the therapy more accessible, reliable, and effective.
Cardiac Resynchronization Therapy Market Segment Overview
By Product Type
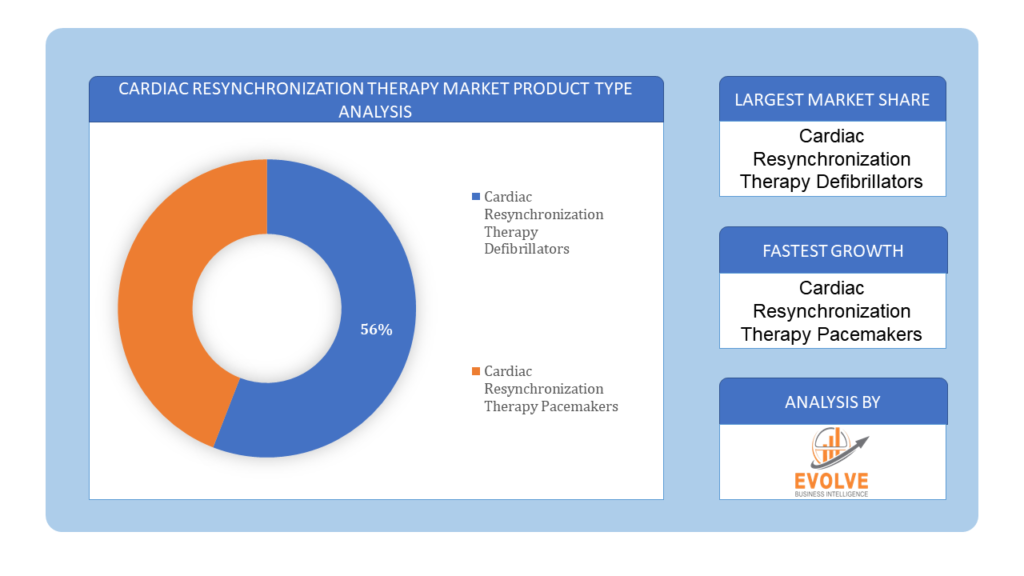 Based on Product Type, the market is segmented based on Cardiac Resynchronization Therapy Defibrillators, Cardiac Resynchronization Therapy Pacemakers. The Cardiac Resynchronization Therapy Defibrillators (CRT-D) segment led the market with the largest share due to its dual functionality in providing cardiac resynchronization therapy and defibrillation capabilities. This integrated approach addresses both heart failure management and arrhythmia prevention, making CRT-D devices essential for patients with advanced heart conditions.
Based on Product Type, the market is segmented based on Cardiac Resynchronization Therapy Defibrillators, Cardiac Resynchronization Therapy Pacemakers. The Cardiac Resynchronization Therapy Defibrillators (CRT-D) segment led the market with the largest share due to its dual functionality in providing cardiac resynchronization therapy and defibrillation capabilities. This integrated approach addresses both heart failure management and arrhythmia prevention, making CRT-D devices essential for patients with advanced heart conditions.
By End User
Based on End User, the market has been divided into the Hospitals & Cardiac Centers, Ambulatory Surgery Centers. The Hospitals & Cardiac Centers segment held the largest market share in the Cardiac Resynchronization Therapy market due to their advanced infrastructure, specialized cardiac care teams, and ability to offer comprehensive treatment options including device implantation and ongoing patient management. These facilities are pivotal in delivering high-quality care and ensuring optimal outcomes for patients requiring cardiac resynchronization therapy.
Global Cardiac Resynchronization Therapy Market Regional Analysis
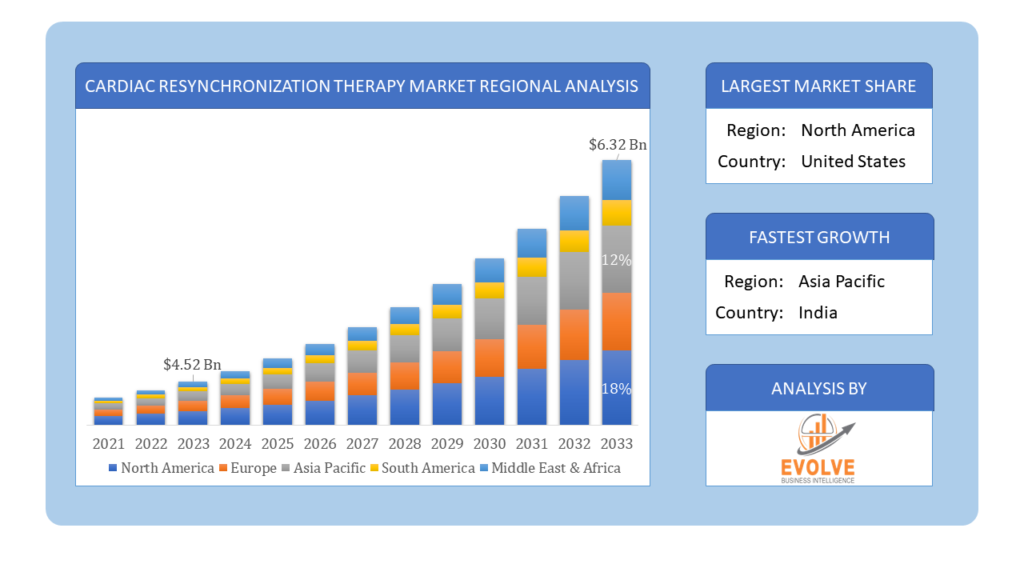
Based on region, the global Cardiac Resynchronization Therapy Market has been divided into North America, Europe, Asia-Pacific, the Middle East & Africa, and Latin America. North America is projected to dominate the use of the Cardiac Resynchronization Therapy Market followed by the Asia-Pacific and Europe regions.
 Cardiac Resynchronization Therapy North America Market
Cardiac Resynchronization Therapy North America Market
North America holds a dominant position in the Cardiac Resynchronization Therapy (CRT) Market due to several key factors. The region benefits from advanced healthcare infrastructure, extensive research and development activities, and a high prevalence of cardiovascular diseases necessitating advanced treatment options like CRT. Moreover, robust healthcare reimbursement policies and a favorable regulatory environment support the adoption of CRT devices. The presence of major market players and ongoing technological innovations further bolster North America’s leadership in the CRT market. Additionally, proactive initiatives by healthcare providers and government agencies to improve patient outcomes and reduce hospital readmissions contribute to the widespread use of CRT therapies across the region.
Cardiac Resynchronization Therapy Asia-Pacific Market
The Asia-Pacific region has indeed emerged as the fastest-growing market for the Cardiac Resynchronization Therapy (CRT) industry due to several compelling factors. Rapid urbanization, increasing healthcare infrastructure investments, and a rising prevalence of cardiovascular diseases contribute to the expanding demand for advanced cardiac care solutions like CRT. Government initiatives aimed at improving healthcare access and quality also drive market growth. Furthermore, advancements in healthcare technology and increasing awareness among healthcare providers and patients about the benefits of CRT therapies propel adoption rates in this region. As a result, Asia-Pacific is witnessing significant market expansion, with both multinational and local companies investing in the development and distribution of CRT devices to meet the growing healthcare needs of its population.
Competitive Landscape
The global Cardiac Resynchronization Therapy Market is highly competitive, with numerous players offering a wide range of software solutions. The competitive landscape is characterized by the presence of established companies, as well as emerging startups and niche players. To increase their market position and attract a wide consumer base, the businesses are employing various strategies, such as product launches, and strategic alliances.
Prominent Players:
- Medtronic
- Abbott
- MicroPort Scientific Corporation
- BIOTRONIK
- Medico S.p.A.
- LivaNova Plc.
- Jude Medical
- Boston Scientific Corporation
- Koninklijke Philips N.V
- Lepu Medical Technology Co. Ltd.
Key Development:
In September 2022, AZ Sint-Jan Brugge-Oostende AV initiated a clinical study to compare the efficacy of two different pacing modalities for cardiac resynchronization therapy.
In September 2022, Assistance Publique-Hôpitaux de Paris launched a clinical study aimed at evaluating cardiac resynchronization therapy in adults with congenital heart disease featuring a systemic right ventricle, focusing on its impact on functional capacity in this patient population.
Scope of the Report
Global Cardiac Resynchronization Therapy Market, by Product Type
- Cardiac Resynchronization Therapy Defibrillators
- Cardiac Resynchronization Therapy Pacemakers
Global Cardiac Resynchronization Therapy Market, by End User
- Hospitals & Cardiac Centers
- Ambulatory Surgery Centers
Global Cardiac Resynchronization Therapy Market, by Region
- North America
- US
- Canada
- Mexico
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Benelux
- Nordic
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- Indonesia
- Austalia
- Malaysia
- India
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of SouthAmerica
- Middle East &Africa
- Saudi Arabia
- UAE
- Egypt
- SouthAfrica
- Rest of Middle East & Africa
| Parameters | Indicators |
|---|---|
| Market Size | 2033: $6.32 Billion |
| CAGR | 5.32% CAGR (2023-2033) |
| Base year | 2022 |
| Forecast Period | 2023-2033 |
| Historical Data | 2021 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, and Trends |
| Key Segmentations | Product Type, End User |
| Geographies Covered | North America, Europe, Asia-Pacific, Latin America, Middle East, Africa |
| Key Vendors | Medtronic, Abbott, MicroPort Scientific Corporation, BIOTRONIK, Medico S.p.A., LivaNova Plc, Jude Medical, Boston Scientific Corporation, Koninklijke Philips N.V, Lepu Medical Technology Co. Ltd. |
| Key Market Opportunities | • Technological Advancements in CRT Devices |
| Key Market Drivers | • Increasing Prevalence of Cardiovascular Diseases
|
REPORT CONTENT BRIEF:
- High-level analysis of the current and future Cardiac Resynchronization Therapy Market trends and opportunities
- Detailed analysis of current market drivers, restraining factors, and opportunities in the future
- Cardiac Resynchronization Therapy Market historical market size for the year 2021, and forecast from 2023 to 2033
- Cardiac Resynchronization Therapy Market share analysis at each product level
- Competitor analysis with detailed insight into its product segment, Government & Defense strength, and strategies adopted.
- Identifies key strategies adopted including product launches and developments, mergers and acquisitions, joint ventures, collaborations, and partnerships as well as funding taken and investment done, among others.
- To identify and understand the various factors involved in the global Cardiac Resynchronization Therapy Market affected by the pandemic
- To provide a detailed insight into the major companies operating in the market. The profiling will include the Government & Defense health of the company’s past 2-3 years with segmental and regional revenue breakup, product offering, recent developments, SWOT analysis, and key strategies.
Press Release

Global Pharmaceutical Manufacturing Market to Reach $1.38 Trillion by 2035 with 7.35% CAGR, New Research Shows

The Global Mammography Market Is Estimated To Record a CAGR of Around 10.29% During The Forecast Period

Glue Stick Market to Reach USD 2.35 Billion by 2034

Podiatry Service Market to Reach USD 11.88 Billion by 2034

Microfluidics Technology Market to Reach USD 32.58 Billion by 2034

Ferric Chloride Market to Reach USD 10.65 Billion by 2034

Family Practice EMR Software Market to Reach USD 21.52 Billion by 2034

Electric Hairbrush Market to Reach USD 15.95 Billion by 2034

Daily Bamboo Products Market to Reach USD 143.52 Billion by 2034

Cross-border E-commerce Logistics Market to Reach USD 112.65 Billion by 2034
Frequently Asked Questions (FAQ)
What is the study period of the Cardiac Resynchronization Therapy Market?
The study period for the Cardiac Resynchronization Therapy Market is from 2023 to 2033.
What is the growth rate of the Cardiac Resynchronization Therapy Market?
The Cardiac Resynchronization Therapy Market is expected to grow at a compound annual growth rate (CAGR) of 5.32% from 2023 to 2033.
Which region has the highest growth rate in the Cardiac Resynchronization Therapy Market?
The Asia-Pacific region has the highest growth rate in the Cardiac Resynchronization Therapy Market, driven by rapid urbanization and increasing healthcare investments.
Which region has the largest share of the Cardiac Resynchronization Therapy Market?
North America holds the largest share of the Cardiac Resynchronization Therapy Market, supported by advanced healthcare infrastructure and a high prevalence of cardiovascular diseases.
Who are the key players in the Cardiac Resynchronization Therapy Market?
Key players in the Cardiac Resynchronization Therapy Market include Medtronic, Abbott, MicroPort Scientific Corporation, BIOTRONIK, and Boston Scientific Corporation.
Do you offer Post sales support?
Yes, we offer 16 hours of analyst support to solve the queries
Do you sell particular sections of a report?
Yes, we provide regional as well as country-level reports. Other than this we also provide a sectional report. Please get in contact with our sales representatives.
Table of Content
Chapter 1. Executive Summary Chapter 2. Scope Of The Study 2.1. Market Definition 2.2. Scope Of The Study 2.2.1. Objectives of Report 2.2.2. Limitations 2.3. Market Structure Chapter 3. Evolve BI Methodology Chapter 4. Market Insights and Trends 4.1. Supply/ Value Chain Analysis 4.1.1. Raw End Users Providers 4.1.2. Manufacturing Process 4.1.3. Distributors/Retailers 4.1.4. End-Use Industry 4.2. Porter’s Five Forces Analysis 4.2.1. Threat Of New Entrants 4.2.2. Bargaining Power Of Buyers 4.2.3. Bargaining Power Of Suppliers 4.2.4. Threat Of Substitutes 4.2.5. Industry Rivalry 4.3. Impact Of COVID-19 on the Cardiac Resynchronization Therapy Market 4.3.1. Impact on Market Size 4.3.2. End-Use Industry Trend, Preferences, and Budget Impact 4.3.3. Regulatory Framework/Government Policies 4.3.4. Key Players' Strategy to Tackle Negative Impact 4.3.5. Opportunity Window 4.4. Technology Overview 12.28. Macro factor 4.6. Micro Factor 4.7.Demand Supply Gap Analysis of the Cardiac Resynchronization Therapy Market 4.8.Import Analysis of the Cardiac Resynchronization Therapy Market 4.9.Export Analysis of the Cardiac Resynchronization Therapy Market Chapter 5. Market Dynamics 5.1. Introduction 5.2. DROC Analysis 5.2.1. Drivers 5.2.2. Restraints 5.2.3. Opportunities 5.2.4. Challenges 5.3. Patent Analysis 5.4. Industry Roadmap 5.5. Parent/Peer Market Analysis Chapter 6. Global Cardiac Resynchronization Therapy Market, By Product Type 6.1. Introduction 6.2. Cardiac Resynchronization Therapy Defibrillators 6.3. Cardiac Resynchronization Therapy Pacemakers Chapter 7. Global Cardiac Resynchronization Therapy Market, By End User 7.1. Introduction 7.2. Hospitals & Cardiac Centers 7.3. Ambulatory Surgery Centers Chapter 8. Global Cardiac Resynchronization Therapy Market, By Region 8.1. Introduction 8.2. North America 8.2.1. Introduction 8.2.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.2.3. Market Size and Forecast, By Country, 2023-2033 8.2.4. Market Size and Forecast, By Product Type, 2023-2033 8.2.5. Market Size and Forecast, By Medtronic, 2023-2033 8.2.6. US 8.2.6.1. Introduction 8.2.6.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.2.6.3. Market Size and Forecast, By Product Type, 2023-2033 8.2.6.4. Market Size and Forecast, By End User, 2023-2033 8.2.7. Canada 8.2.7.1. Introduction 8.2.7.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.2.7.4. Market Size and Forecast, By Product Type, 2023-2033 8.2.7.5. Market Size and Forecast, By End User, 2023-2033 8.3. Europe 8.3.1. Introduction 8.3.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.3. Market Size and Forecast, By Country, 2023-2033 8.3.4. Market Size and Forecast, By Product Type, 2023-2033 8.3.5. Market Size and Forecast, By End User, 2023-2033 8.3.6. Germany 8.3.6.1. Introduction 8.3.6.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.6.3. Market Size and Forecast, By Product Type, 2023-2033 8.3.6.4. Market Size and Forecast, By End User, 2023-2033 8.3.7. France 8.3.7.1. Introduction 8.3.7.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.7.3. Market Size and Forecast, By Product Type, 2023-2033 8.3.7.4. Market Size and Forecast, By End User, 2023-2033 8.3.8. UK 8.3.8.1. Introduction 8.3.8.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.8.3. Market Size and Forecast, By Product Type, 2023-2033 8.3.8.4. Market Size and Forecast, By End User, 2023-2033 8.3.9. Italy 8.3.9.1. Introduction 8.3.9.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.9.3. Market Size and Forecast, By Product Type, 2023-2033 8.3.9.4. Market Size and Forecast, By End User, 2023-2033 8.3.11. Rest Of Europe 8.3.11.1. Introduction 8.3.11.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.11.3. Market Size and Forecast, By Product Type, 2023-2033 8.3.11.4. Market Size and Forecast, By End User, 2023-2033 8.4. Asia-Pacific 8.4.1. Introduction 8.4.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.3. Market Size and Forecast, By Country, 2023-2033 8.4.4. Market Size and Forecast, By Product Type, 2023-2033 8.12.28. Market Size and Forecast, By End User, 2023-2033 8.4.6. China 8.4.6.1. Introduction 8.4.6.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.6.3. Market Size and Forecast, By Product Type, 2023-2033 8.4.6.4. Market Size and Forecast, By End User, 2023-2033 8.4.7. India 8.4.7.1. Introduction 8.4.7.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.7.3. Market Size and Forecast, By Product Type, 2023-2033 8.4.7.4. Market Size and Forecast, By End User, 2023-2033 8.4.8. Japan 8.4.8.1. Introduction 8.4.8.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.8.3. Market Size and Forecast, By Product Type, 2023-2033 8.4.8.4. Market Size and Forecast, By End User, 2023-2033 8.4.9. South Korea 8.4.9.1. Introduction 8.4.9.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.9.3. Market Size and Forecast, By Product Type, 2023-2033 8.4.9.4. Market Size and Forecast, By End User, 2023-2033 8.4.10. Rest Of Asia-Pacific 8.4.10.1. Introduction 8.4.10.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.10.3. Market Size and Forecast, By Product Type, 2023-2033 8.4.10.4. Market Size and Forecast, By End User, 2023-2033 8.5. Rest Of The World (RoW) 8.5.1. Introduction 8.5.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.5.3. Market Size and Forecast, By Product Type, 2023-2033 8.5.4. Market Size and Forecast, By End User, 2023-2033 Chapter 9. Company Landscape 9.1. Introduction 9.2. Vendor Share Analysis 9.3. Key Development Analysis 9.4. Competitor Dashboard Chapter 10. Company Profiles 10.1. Medtronic 10.1.1. Business Overview 10.1.2. Government & Defense Analysis 10.1.2.1. Government & Defense – Existing/Funding 10.1.3. Product Portfolio 10.1.4. Recent Development and Strategies Adopted 10.1.5. SWOT Analysis 10.2. Abbott 10.2.1. Business Overview 10.2.2. Government & Defense Analysis 10.2.2.1. Government & Defense – Existing/Funding 10.2.3. Product Portfolio 10.2.4. Recent Development and Strategies Adopted 10.2.5. SWOT Analysis 10.3. MicroPort Scientific Corporation 10.3.1. Business Overview 10.3.2. Government & Defense Analysis 10.3.2.1. Government & Defense – Existing/Funding 10.3.3. Product Portfolio 10.3.4. Recent Development and Strategies Adopted 10.3.5. SWOT Analysis 10.4. BIOTRONIK 10.4.1. Business Overview 10.4.2. Government & Defense Analysis 10.4.2.1. Government & Defense – Existing/Funding 10.4.3. Product Portfolio 10.4.4. Recent Development and Strategies Adopted 10.12.28. SWOT Analysis 10.5. Medico S.p.A. 10.5.1. Business Overview 10.5.2. Government & Defense Analysis 10.5.2.1. Government & Defense – Existing/Funding 10.5.3. Product Portfolio 10.5.4. Recent Development and Strategies Adopted 10.5.5. SWOT Analysis 10.6. LivaNova Plc. 10.6.1. Business Overview 10.6.2. Government & Defense Analysis 10.6.2.1. Government & Defense – Existing/Funding 10.6.3. Product Portfolio 10.6.4. Recent Development and Strategies Adopted 10.6.5. SWOT Analysis 10.7. Jude Medical 10.7.1. Business Overview 10.7.2. Government & Defense Analysis 10.7.2.1. Government & Defense – Existing/Funding 10.7.3. Product Portfolio 10.7.4. Recent Development and Strategies Adopted 10.7.5. SWOT Analysis 10.8 Boston Scientific Corporation 10.8.1. Business Overview 10.8.2. Government & Defense Analysis 10.8.2.1. Government & Defense – Existing/Funding 10.8.3. Product Portfolio 10.8.4. Recent Development and Strategies Adopted 10.8.5. SWOT Analysis 10.9 Koninklijke Philips N.V 10.9.1. Business Overview 10.9.2. Government & Defense Analysis 10.9.2.1. Government & Defense – Existing/Funding 10.9.3. Product Portfolio 10.9.4. Recent Development and Strategies Adopted 10.9.5. SWOT Analysis 10.10. Lepu Medical Technology Co. Ltd. 10.10.1. Business Overview 10.10.2. Government & Defense Analysis 10.10.2.1. Government & Defense – Existing/Funding 10.10.3. Product Portfolio 10.10.4. Recent Development and Strategies Adopted 10.10.5. SWOT Analysis
Connect to Analyst
Research Methodology