Gene Therapy Market Analysis and Global Forecast 2023-2033
€ 1,230.43 – € 4,886.30Price range: € 1,230.43 through € 4,886.30
Gene Therapy Market Research Report: Information By Therapy Type (Somatic, Germline), By Vector Type (Non-Viral Vectors, Viral Vectors), By Application (Cancer Diseases, Monogenic Diseases, Infectious Diseases, Cardiovascular Diseases, Others), and by Region — Forecast till 2033
Gene Therapy Market Overview
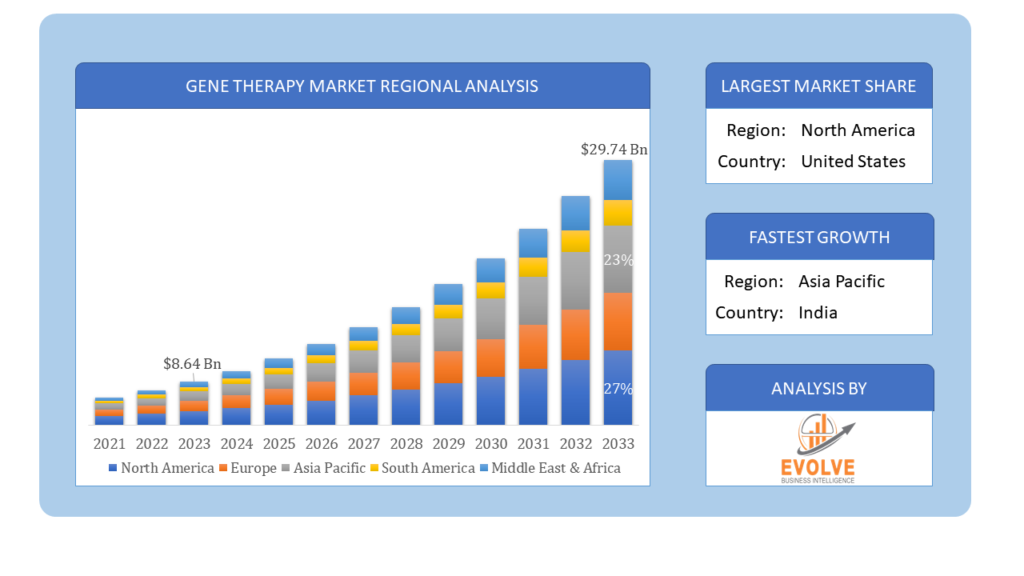
The Gene Therapy Market Size is expected to reach USD 29.74 Billion by 2033. The Gene Therapy industry size accounted for USD 8.64 Billion in 2023 and is expected to expand at a compound annual growth rate (CAGR) of 19.1% from 2023 to 2033. Gene therapy represents a cutting-edge medical approach that seeks to treat or prevent genetic disorders, diseases, or acquired conditions by introducing, modifying, or replacing genetic material within a person’s cells. This groundbreaking technique aims to rectify defective genes through the delivery of therapeutic genes directly to affected tissues or by using modified viruses as carriers. By addressing the root genetic causes at the molecular level, gene therapy holds tremendous potential for curing previously incurable conditions. Nonetheless, it remains an evolving field of research, necessitating comprehensive evaluation, ethical deliberation, and meticulous long-term monitoring to ensure its wide-ranging application is safe and effective.
Global Gene Therapy Market Synopsis
The Gene Therapy market underwent a profound and transformative shift due to the impact of the COVID-19 pandemic. With the world confronting an unprecedented health crisis, there was an urgent demand for effective treatments and vaccines, spurring accelerated research and development in gene therapy. The pandemic served as a catalyst, shedding light on the immense potential of this innovative approach in providing targeted therapies and immunization strategies. Moreover, it led to increased investment in biotechnology and gene therapy companies, resulting in a surge of funding and collaborations to expedite the progress of gene-based solutions. Additionally, the pandemic highlighted the significance of regulatory advancements and streamlined approval processes for gene therapies, facilitating quicker access to potentially life-saving treatments.
Gene Therapy Market Dynamics
The major factors that have impacted the growth of Gene Therapy are as follows:
Drivers:
Ø Advancements in Biotechnology and Gene Editing Techniques
The rapid progress in biotechnology and gene editing techniques, such as CRISPR-Cas9, has significantly improved the precision and efficiency of gene therapy. These advancements have enabled researchers to develop more targeted and effective gene therapies, increasing the potential for successful treatments and expanding the scope of gene therapy applications.
Restraint:
- High Development Costs and Pricing Challenges
Gene therapy development involves complex research, preclinical studies, clinical trials, and regulatory processes, which can result in high development costs. Additionally, pricing challenges arise as gene therapies may be costly due to the sophisticated manufacturing processes and limited patient populations for rare genetic disorders. These high costs and pricing pressures can limit patient access and reimbursement, impacting the widespread adoption of gene therapies.
Opportunity:
⮚ Potential Cures for Previously Untreatable Conditions
Gene therapy offers the potential to cure or significantly alleviate previously untreatable genetic disorders and diseases. By addressing the root causes of these conditions at the genetic level, gene therapy has the opportunity to provide long-lasting and transformative benefits to patients who had limited treatment options before, opening new avenues for healthcare improvement and patient outcomes.
Gene Therapy Segment Overview
By Therapy Type
 Based on Therapy Type, the market is segmented based on Somatic, Germline. The Somatic segment is poised for significant expansion, driving growth in the Gene Therapy market during the forecast period, fueled by advancing technologies and increasing clinical applications.
Based on Therapy Type, the market is segmented based on Somatic, Germline. The Somatic segment is poised for significant expansion, driving growth in the Gene Therapy market during the forecast period, fueled by advancing technologies and increasing clinical applications.
By Vector Type
Based on Vector Type, the market has been divided into Non-Viral Vectors, Viral Vectors. Non-viral vectors emerge as the predominant force in the Gene Therapy Market, fueled by their versatility, safety profiles, and potential for large-scale production, driving innovation and adoption in therapeutic applications.
By Application
Based on Application, the market has been divided into Cancer Diseases, Monogenic Diseases, Infectious Diseases, Cardiovascular Diseases, Others. Cancer diseases assert dominance in the Gene Therapy Market, spearheading research and investment due to the urgent need for effective treatments, propelling advancements in targeted therapies and immunotherapies for various cancer types.
Global Gene Therapy Market Regional Analysis
Based on region, the global Gene Therapy market has been divided into North America, Europe, Asia-Pacific, the Middle East & Africa, and Latin America. North America is projected to dominate the use of the Gene Therapy market followed by the Asia-Pacific and Europe regions.
 Gene Therapy North America Market
Gene Therapy North America Market
North America has consistently maintained a dominant position in the Gene Therapy market. This is attributed to several factors, including robust investments in research and development, a well-established biotechnology and pharmaceutical industry, and supportive regulatory frameworks that facilitate the advancement of gene therapies. The region’s strong infrastructure for clinical trials and healthcare, along with a high prevalence of genetic disorders and chronic diseases, has encouraged extensive gene therapy research and adoption. Furthermore, North America has witnessed significant collaborations between academia, industry, and government agencies, fostering innovation and accelerating the translation of gene therapy discoveries into clinical applications. The region’s leadership in gene therapy has led to the successful approval and commercialization of several gene-based treatments, consolidating its dominant position in this rapidly evolving market.
Gene Therapy Asia-Pacific Market
The Asia-Pacific region has emerged as a rapidly growing market for the Gene Therapy industry. This growth can be attributed to various factors that have contributed to the region’s increasing prominence in the field. Firstly, the region has witnessed significant investments in biotechnology and life sciences research, fostering the development of advanced gene therapy technologies. Secondly, a large and diverse patient population with a high prevalence of genetic disorders and chronic diseases has created a substantial demand for innovative treatment options, driving the adoption of gene therapies. Additionally, favorable regulatory environments in some countries within the region have facilitated the approval and commercialization of gene therapies, enabling quicker market access for these cutting-edge treatments. Moreover, collaborations between local research institutions and international biotechnology companies have accelerated research efforts and enhanced expertise in gene therapy, further fueling the region’s growth as a prominent player in the global Gene Therapy market.
Competitive Landscape
The Global Gene Therapy market is highly competitive, with numerous players offering a wide range of software solutions. The competitive landscape is characterized by the presence of established companies, as well as emerging startups and niche players. To increase their market position and attract a wide consumer base, the businesses are employing various strategies, such as product launches, and strategic alliances.
Prominent Players:
- REGENXBIO Inc
- Oxford BioMedica plc
- Dimension Therapeutics Inc
- Bristol-Myers Squibb Company
- SANOFI
- Applied Genetic Technologies Corporation
- Hoffmann-La Roche Ltd
- bluebird Bio Inc
- Novartis AG
- Taxus Cardium Pharmaceuticals Group Inc
Key Development
In October 2022, Sarepta Therapeutics applied to the US FDA, seeking accelerated approval for the gene therapy S (delandistrogene moxeparvovec) as a treatment for Duchenne Muscular Dystrophy (DMD).
In August 2022, the US FDA approved Zynteglo (betibeglogene autotemcel), making it the first-ever cell-based gene therapy to be approved for the treatment of beta-thalassemia in both adult and pediatric patients who require regular red blood cell transfusions.
Scope of the Report
Global Gene Therapy Market, by Therapy Type
- Somatic
- Germline
Global Gene Therapy Market, by Vector Type
- Non-Viral Vectors
- Viral Vectors
Global Gene Therapy Market, by Application
- Cancer Diseases
- Monogenic Diseases
- Infectious Diseases
- Cardiovascular Diseases
- Others
Global Gene Therapy Market, by Region
- North America
- US
- Canada
- Mexico
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Benelux
- Nordic
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- Indonesia
- Austalia
- Malaysia
- India
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- South Africa
- Rest of Middle East & Africa
| Parameters | Indicators |
|---|---|
| Market Size | 2033: $29.74 Billion |
| CAGR | 19.1% CAGR (2023-2033) |
| Base year | 2022 |
| Forecast Period | 2023-2033 |
| Historical Data | 2021 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, and Trends |
| Key Segmentations | Type, Component, Technology, Application, Location, Size |
| Geographies Covered | North America, Europe, Asia-Pacific, Latin America, Middle East, Africa |
| Key Vendors | REGENXBIO Inc, Oxford BioMedica plc, Dimension Therapeutics Inc, Bristol-Myers Squibb Company, SANOFI, Applied Genetic Technologies Corporation, F. Hoffmann-La Roche Ltd, bluebird Bio Inc, Novartis AG, Taxus Cardium Pharmaceuticals Group Inc |
| Key Market Opportunities | • The increasing prevalence of genetic disorders and chronic diseases |
| Key Market Drivers | • Advancements in gene editing techniques |
REPORT CONTENT BRIEF:
- High-level analysis of the current and future Gene Therapy market trends and opportunities
- Detailed analysis of current market drivers, restraining factors, and opportunities in the future
- Gene Therapy market historical market size for the year 2021, and forecast from 2023 to 2033
- Gene Therapy market share analysis at each product level
- Competitor analysis with detailed insight into its product segment, Government & Defense strength, and strategies adopted.
- Identifies key strategies adopted including product launches and developments, mergers and acquisitions, joint ventures, collaborations, and partnerships as well as funding taken and investment done, among others.
- To identify and understand the various factors involved in the global Gene Therapy market affected by the pandemic
- To provide a detailed insight into the major companies operating in the market. The profiling will include the Government & Defense health of the company’s past 2-3 years with segmental and regional revenue breakup, product offering, recent developments, SWOT analysis, and key strategies.
Press Release

Global Pharmaceutical Manufacturing Market to Reach $1.38 Trillion by 2035 with 7.35% CAGR, New Research Shows

The Global Mammography Market Is Estimated To Record a CAGR of Around 10.29% During The Forecast Period

Glue Stick Market to Reach USD 2.35 Billion by 2034

Podiatry Service Market to Reach USD 11.88 Billion by 2034

Microfluidics Technology Market to Reach USD 32.58 Billion by 2034

Ferric Chloride Market to Reach USD 10.65 Billion by 2034

Family Practice EMR Software Market to Reach USD 21.52 Billion by 2034

Electric Hairbrush Market to Reach USD 15.95 Billion by 2034

Daily Bamboo Products Market to Reach USD 143.52 Billion by 2034

Cross-border E-commerce Logistics Market to Reach USD 112.65 Billion by 2034
Frequently Asked Questions (FAQ)
What is the projected size of the Gene Therapy market by 2033?
The Gene Therapy market is expected to reach USD 29.74 Billion by 2033, with a compound annual growth rate (CAGR) of 19.1% from 2023 to 2033
What are the major factors driving the growth of the Gene Therapy market?
Advancements in biotechnology and gene editing techniques, such as CRISPR-Cas9, have significantly improved the precision and efficiency of gene therapy, expanding its potential applications
What are the challenges hindering the widespread adoption of gene therapies?
High development costs and pricing challenges due to complex research, clinical trials, and limited patient populations for rare genetic disorders can limit patient access and reimbursement, impacting adoption
Which region is projected to dominate the Gene Therapy market?
North America is expected to dominate the Gene Therapy market, followed by the Asia-Pacific and Europe regions, attributed to robust investments in research and development, supportive regulatory frameworks, and a high prevalence of genetic disorders
Who are the prominent players in the Gene Therapy market?
Prominent players in the Gene Therapy market include REGENXBIO Inc, Oxford BioMedica plc, Dimension Therapeutics Inc, Bristol-Myers Squibb Company, SANOFI, among others, employing strategies like product launches and strategic alliances to enhance market position
Do you offer Post sales support?
Yes, we offer 16 hours of analyst support to solve the queries
Do you sell particular sections of a report?
Yes, we provide regional as well as country-level reports. Other than this we also provide a sectional report. Please get in contact with our sales representatives.
Table of Content
CHAPTER 1. Executive Summary CHAPTER 2. Scope of the Study 2.1. Market Definition 2.2. Market Scope & Segmentation 2.2.1. Objective of Report CHAPTER 3. Evolve BI Methodology 3.1. Data Collection & Validation Approach 3.2. Market Size Estimation and Forecast CHAPTER 4. Exclusive Analysis 4.1. Market Opportunity Score 4.1.1. Therapy Type Segement – Market Opportunity Score 4.1.2. Vector Type Segment – Market Opportunity Score 4.1.3. Application Segment – Market Opportunity Score 4.2. Key Market Influencing Indicators CHAPTER 5. Market Insights and Trends 5.1. Value Chain Analysis 5.1.1. Raw Material 5.1.2. Manufacturing Process 5.1.3. Distribution Channel 5.1.4. End User 5.2. Porter’s Five Forces Analysis 5.2.1. Bargaining Power of Buyers 5.2.2. Bargaining Power of Suppliers 5.2.3. Threat of New Entrant 5.2.4. Threat of Substitute 5.2.5. Industry Rivalry 5.3. COVID-19 Impact and Post COVID Scenario on Gene Therapy Market 5.3.1. Impact of COVID-19 5.3.2. Government Support and Industry Revival Policies 5.3.3. Measures Taken by Companies to Mitigate Negative Impact 5.3.4. Post COVID Trend CHAPTER 6. MArket Dynamics 6.1. Introduction 6.2. Drivers 6.2.1. Driver 1 6.2.2. Driver 2 6.2.3. Driver 3 6.3. Restraints 6.3.1. Restraint 1 6.3.2. Restraint 2 6.4. Opportunity 6.4.1. Opportunity 1 CHAPTER 7. Global Gene Therapy Market, By Therapy Type 7.1. Introduction 7.1.1. Somatic 7.1.2. Germline CHAPTER 8. Global Gene Therapy Market, By Vector Type 8.1. Introduction 8.1.1. Non-Viral Vectors 8.1.2. Viral Vectors CHAPTER 9. Global Gene Therapy Market, By Application 9.1. Introduction 9.1.1. Cancer Diseases 9.1.2. Monogenic Diseases 9.1.3. Infectious Diseases 9.1.4. Cardiovascular Diseases 9.1.5. Others CHAPTER 10. Global Gene Therapy Market, By Region 10.1. Introduction 10.2. NORTH AMERICA 10.2.1. North America: Market Size and Forecast, By Country, 2023 – 2033 ($ Million) 10.2.2. North America: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.2.3. North America: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.2.4. North America: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.2.5. US 10.2.5.1. US: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.2.5.2. US: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.2.5.3. US: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.2.6. CANADA 10.2.6.1. Canada: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.2.6.2. Canada: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.2.6.3. Canada: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.2.7. MEXICO 10.2.7.1. Mexico: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.2.7.2. Mexico: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.2.7.3. Mexico: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.3. Europe 10.3.1. Europe: Market Size and Forecast, By Country, 2023 – 2033 ($ Million) 10.3.2. Europe: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.3.3. Europe: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.3.4. Europe: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.3.5. U.K. 10.3.5.1. U.K.: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.3.5.2. U.K.: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.3.5.3. U.K.: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.3.6. GERMANY 10.3.6.1. Germany: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.3.6.2. Germany: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.3.6.3. Germany: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.3.7. FRANCE 10.3.7.1. France: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.3.7.2. France: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.3.7.3. France: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.3.8. ITALY 10.3.8.1. Italy: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.3.8.2. Italy: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.3.8.3. Italy: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.3.9. SPAIN 10.3.9.1. Spain: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.3.9.2. Spain: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.3.9.3. Spain: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.3.10. BENELUX 10.3.10.1. BeNeLux: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.3.10.2. BeNeLux: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.3.10.3. BeNeLux: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.3.11. RUSSIA 10.3.11.1. Russia: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.3.11.2. Russia: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.3.11.3. Russia: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.3.12. REST OF EUROPE 10.3.12.1. Rest of Europe: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.3.12.2. Rest of Europe: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.3.12.3. Rest of Europe: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.4. Asia Pacific 10.4.1. Asia Pacific: Market Size and Forecast, By Country, 2023 – 2033 ($ Million) 10.4.2. Asia Pacific: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.4.3. Asia Pacific: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.4.4. Asia Pacific: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.4.5. CHINA 10.4.5.1. China: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.4.5.2. China: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.4.5.3. China: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.4.6. JAPAN 10.4.6.1. Japan: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.4.6.2. Japan: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.4.6.3. Japan: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.4.7. INDIA 10.4.7.1. India: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.4.7.2. India: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.4.7.3. India: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.4.8. SOUTH KOREA 10.4.8.1. South Korea: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.4.8.2. South Korea: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.4.8.3. South Korea: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.4.9. THAILAND 10.4.9.1. Thailand: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.4.9.2. Thailand: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.4.9.3. Thailand: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.4.10. INDONESIA 10.4.10.1. Indonesia: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.4.10.2. Indonesia: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.4.10.3. Indonesia: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.4.11. MALAYSIA 10.4.11.1. Malaysia: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.4.11.2. Malaysia: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.4.11.3. Malaysia: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.4.12. AUSTRALIA 10.4.12.1. Australia: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.4.12.2. Australia: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.4.12.3. Australia: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.4.13. REST FO ASIA PACIFIC 10.4.13.1. Rest fo Asia Pacific: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.4.13.2. Rest fo Asia Pacific: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.4.13.3. Rest fo Asia Pacific: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.5. South America 10.5.1. South America: Market Size and Forecast, By Country, 2023 – 2033 ($ Million) 10.5.2. South America: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.5.3. South America: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.5.4. South America: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.5.5. BRAZIL 10.5.5.1. Brazil: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.5.5.2. Brazil: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.5.5.3. Brazil: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.5.6. ARGENTINA 10.5.6.1. Argentina: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.5.6.2. Argentina: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.5.6.3. Argentina: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.5.7. REST OF SOUTH AMERICA 10.5.7.1. Rest of South America: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.5.7.2. Rest of South America: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.5.7.3. Rest of South America: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.6. Middle East & Africa 10.6.1. Middle East & Africa: Market Size and Forecast, By Country, 2023 – 2033 ($ Million) 10.6.2. Middle East & Africa: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.6.3. Middle East & Africa: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.6.4. Middle East & Africa: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.6.5. SAUDI ARABIA 10.6.5.1. Saudi Arabia: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.6.5.2. Saudi Arabia: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.6.5.3. Saudi Arabia: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.6.6. UAE 10.6.6.1. UAE: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.6.6.2. UAE: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.6.6.3. UAE: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.6.7. EGYPT 10.6.7.1. Egypt: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.6.7.2. Egypt: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.6.7.3. Egypt: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.6.8. SOUTH AFRICA 10.6.8.1. South Africa: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.6.8.2. South Africa: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.6.8.3. South Africa: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) 10.6.9. REST OF MIDDLE EAST & AFRICA 10.6.9.1. Rest of Middle East & Africa: Market Size and Forecast, By Therapy Type, 2023 – 2033 ($ Million) 10.6.9.2. Rest of Middle East & Africa: Market Size and Forecast, By Vector Type, 2023 – 2033 ($ Million) 10.6.9.3. Rest of Middle East & Africa: Market Size and Forecast, By Application, 2023 – 2033 ($ Million) CHAPTER 12. Competitive Landscape 12.1. Competitior Benchmarking 2023 12.2. Market Share Analysis 12.3. Key Developments Analysis By Top 5 Companies 12.4. Market Share Acquisition Strategies: Analysis of Key Approaches Employed by Top Players CHAPTER 13. Company Profiles 13.1. REGENXBIO Inc 13.1.1. Business Overview 13.1.2. Financial Analysis 13.1.2.1. Business Segment Revenue, 2020, 2021, 2022, $ Million 13.1.2.2. Geographic Revenue Mix, 2022 (% Share) 13.1.3. Product Portfolio 13.1.4. Recent Development and Strategies Adopted 13.1.5. SWOT Analysis 13.2. Oxford BioMedica plc 13.3. Dimension Therapeutics Inc 13.4. Bristol-Myers Squibb Company 13.5. SANOFI 13.6. Applied Genetic Technologies Corporation. 13.7. Hoffmann-La Roche Ltd 13.8. bluebird Bio Inc 13.9. Novartis AG 13.10. Taxus Cardium Pharmaceuticals Group Inc
Connect to Analyst
Research Methodology