Epilepsy Devices Market Analysis and Global Forecast 2023-2033
$ 1,390.00 – $ 5,520.00Price range: $ 1,390.00 through $ 5,520.00
Epilepsy Devices Market Research Report: Information By Product Type (Conventional Devices, Wearable Devices, Others), By End-Users (Hospitals & Clinics, Home Care Settings, Others), and by Region — Forecast till 2033
Page: 125
Epilepsy Devices Market Overview
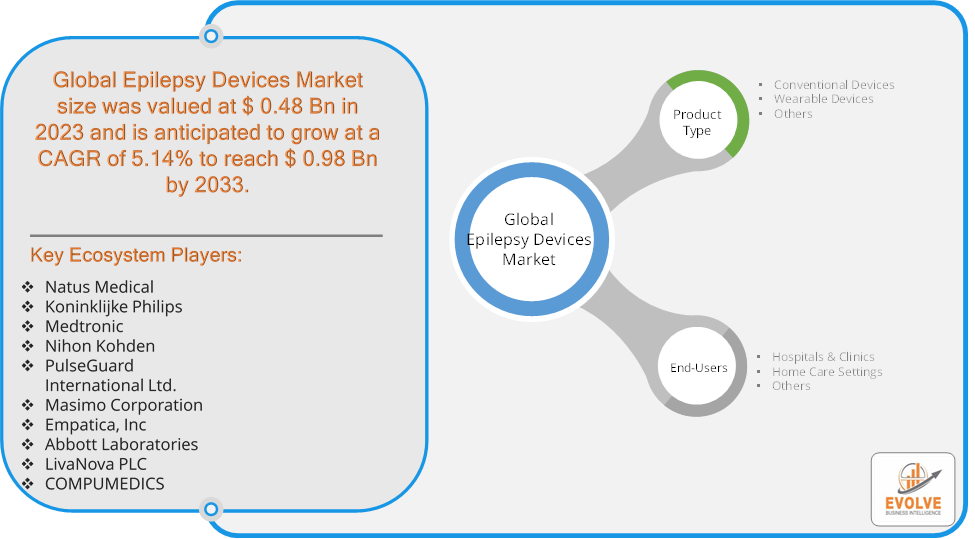
The Epilepsy Devices Market Size is expected to reach USD 0.98 Billion by 2033. The Epilepsy Devices industry size accounted for USD 0.48 Billion in 2023 and is expected to expand at a compound annual growth rate (CAGR) of 5.14% from 2023 to 2033. The epilepsy devices market encompasses products designed to manage and treat epilepsy, a neurological disorder characterized by recurrent seizures. Key devices in this market include neurostimulation devices (such as responsive neurostimulation and deep brain stimulation systems), wearable seizure monitors, and EEG (electroencephalogram) devices. These technologies aim to detect, predict, or control seizures, improving patients’ quality of life and reducing the frequency of seizures. The market is driven by advancements in technology, increasing prevalence of epilepsy, and growing awareness of epilepsy management options. It is influenced by factors such as innovation in device design, regulatory approvals, and the expansion of healthcare infrastructure.
Global Epilepsy Devices Market Synopsis
 COVID-19 Impact Analysis
COVID-19 Impact Analysis
The COVID-19 pandemic has led to supply chain disruptions leading to supply shortages or lower demand in the Epilepsy Devices market. The travel restrictions and social-distancing measures have resulted in a sharp drop in consumer and business spending and this pattern is to continue for some time. The end-user trend and preferences have changed due to the pandemic and have resulted in manufacturers, developers, and service providers to adopt various strategies to stabilize the company.
Epilepsy Devices Market Dynamics
The major factors that have impacted the growth of Epilepsy Devices are as follows:
Drivers:
Ø Technological Advancements
Innovations in neurostimulation technology, such as responsive neurostimulation (RNS) and deep brain stimulation (DBS), have significantly enhanced the efficacy and safety of epilepsy treatment. Wearable devices and advanced EEG systems also contribute by providing more precise seizure detection and monitoring. Continuous improvements in device technology drive market growth by offering better outcomes and more options for patients.
Restraint:
- Technological Limitations and Reliability Issues
While technology has advanced, some epilepsy devices may still face issues related to reliability, accuracy, and ease of use. Problems such as false alarms, device malfunctions, or inadequate performance can undermine patient trust and affect the overall effectiveness of the devices. Ensuring consistent reliability is crucial for market success.
Opportunity:
⮚ Technological Limitations
Some epilepsy devices may face issues related to reliability, accuracy, and usability. Problems such as false positives or negatives, device malfunctions, or user difficulties can undermine their effectiveness and patient trust. Ensuring that devices perform reliably and are user-friendly is crucial for their success in the market.
Epilepsy Devices Segment Overview
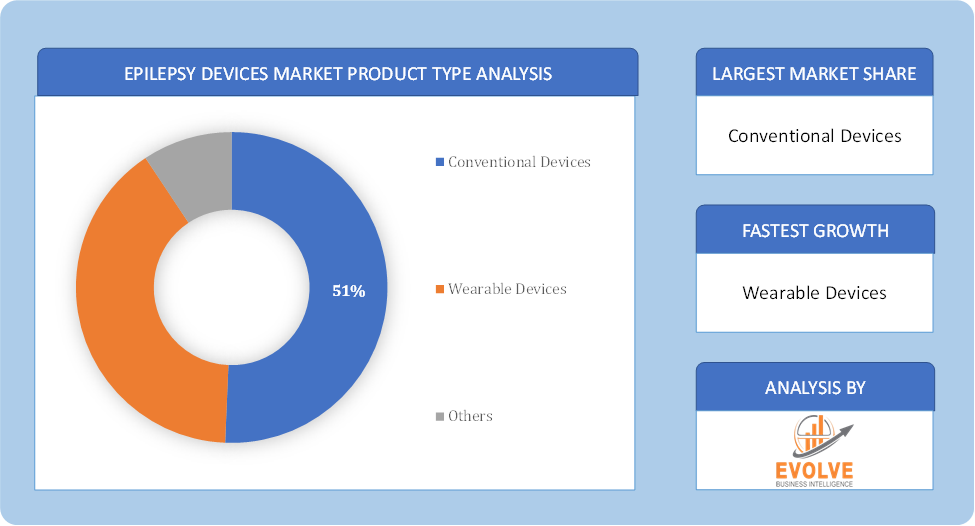 By Product Type
By Product Type
Based on Product Type, the market is segmented based on Conventional Devices, Wearable Devices, Others. conventional devices dominate due to their established use and effectiveness in managing epilepsy through methods like neurostimulation and traditional monitoring, despite the growing interest in innovative wearable devices.
By End User
Based on End Users, the market has been divided into the Hospitals & Clinics, Home Care Settings, Others. hospitals and clinics dominate due to their advanced infrastructure and specialized medical teams, which are essential for the installation, monitoring, and maintenance of sophisticated epilepsy devices.
Global Epilepsy Devices Market Regional Analysis
Based on region, the global Epilepsy Devices market has been divided into North America, Europe, Asia-Pacific, the Middle East & Africa, and Latin America. North America is projected to dominate the use of the Epilepsy Devices market followed by the Asia-Pacific and Europe regions.
 Epilepsy Devices North America Market
Epilepsy Devices North America Market
North America holds a dominant position in the Epilepsy Devices Market. North America is anticipated to continue to dominate the market for epilepsy devices during the forecast period, holding a sizeable portion of the market share in 2021. In addition, the rising number of patients receiving neurosurgical treatments in this area supports the market’s expansion. In addition, strong purchasing power and a rise in the use of epilepsy devices are anticipated to propel market expansion.
Epilepsy Devices Asia-Pacific Market
The Asia-Pacific region has indeed emerged as the fastest-growing market for the Epilepsy Devices industry. From 2022 to2030, the epilepsy devices market in Asia-Pacific is anticipated to expand at the quickest rate (CAGR). This is because there are medical equipment companies in the area and because populated countries like China and India have increasing purchasing power. Additionally, the market is expanding due to rising healthcare costs and growing usage of epilepsy gadgets including accelerometers, surface electromyography, and EEG systems.
Competitive Landscape
The global Epilepsy Devices market is highly competitive, with numerous players offering a wide range of software solutions. The competitive landscape is characterized by the presence of established companies, as well as emerging startups and niche players. To increase their market position and attract a wide consumer base, the businesses are employing various strategies, such as product launches, and strategic alliances.
Prominent Players:
- Natus Medical
- Koninklijke Philips
- Medtronic
- Nihon Kohden
- PulseGuard International Ltd.
- Masimo Corporation
- Empatica, Inc
- Abbott Laboratories
- LivaNova PLC
- COMPUMEDICS
Key Development
In February 2019, Philips teamed with researchers from Eindhoven University of Technology, Kempenhaeghe, and Ghent University in Belgium to develop a method to stimulate the brain using electrodes put on the head rather than inside it. Their goal is to tailor treatment for those suffering from severe epilepsy.
Scope of the Report
Global Epilepsy Devices Market, by Product Type
- Conventional Devices
- Wearable Devices
- Others
Global Epilepsy Devices Market, by End-Users
- Hospitals & Clinics
- Home Care Settings
- Others
Global Epilepsy Devices Market, by Region
- North America
- US
- Canada
- Mexico
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Benelux
- Nordic
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- Indonesia
- Austalia
- Malaysia
- India
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- South Africa
- Rest of Middle East & Africa
| Parameters | Indicators |
|---|---|
| Market Size | 2033: USD 0.98Billion |
| CAGR (2023-2033) | 5.14% |
| Base year | 2022 |
| Forecast Period | 2023-2033 |
| Historical Data | 2021 (2017 to 2020 On Demand) |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, and Trends |
| Key Segmentations | Product Type, End User |
| Geographies Covered | North America, Europe, Asia-Pacific, South America, Middle East, Africa |
| Key Vendors | Natus Medical, Koninklijke Philips, Medtronic, Nihon Kohden, PulseGuard International Ltd., Masimo Corporation, Empatica, Inc, Abbott Laboratories, LivaNova PLC, COMPUMEDICS. |
| Key Market Opportunities | · New markets for gadgets used to treat epilepsy |
| Key Market Drivers | · Growing occurrences of brain damage from car accidents and rising rates of epilepsy among the elderly population |
REPORT CONTENT BRIEF:
- High-level analysis of the current and future Epilepsy Devices market trends and opportunities
- Detailed analysis of current market drivers, restraining factors, and opportunities in the future
- Epilepsy Devices market historical market size for the year 2021, and forecast from 2023 to 2033
- Epilepsy Devices market share analysis at each product level
- Competitor analysis with detailed insight into its product segment, Government & Defense strength, and strategies adopted.
- Identifies key strategies adopted including product launches and developments, mergers and acquisitions, joint ventures, collaborations, and partnerships as well as funding taken and investment done, among others.
- To identify and understand the various factors involved in the global Epilepsy Devices market affected by the pandemic
- To provide a detailed insight into the major companies operating in the market. The profiling will include the Government & Defense health of the company’s past 2-3 years with segmental and regional revenue breakup, product offering, recent developments, SWOT analysis, and key strategies.
Frequently Asked Questions (FAQ)
What is the study period of this market?
The study period of the global Epilepsy Devices market is 2021- 2033
What is the growth rate of the global Epilepsy Devices market?
The global Epilepsy Devices market is growing at a CAGR of 5.14% over the next 10 years
Which region has the highest growth rate in the market of Epilepsy Devices?
Asia Pacific is expected to register the highest CAGR during 2023-2033
Which region has the largest share of the global Epilepsy Devices market?
North America holds the largest share in 2022
Who are the key players in the global Epilepsy Devices market?
Natus Medical, Koninklijke Philips, Medtronic, Nihon Kohden, PulseGuard International Ltd., Masimo Corporation, Empatica, Inc, Abbott Laboratories, LivaNova PLC, and COMPUMEDICS are the major companies operating in the market.
Do you offer Post Sale Support?
Yes, we offer 16 hours of analyst support to solve the queries
Do you sell particular sections of a report?
Yes, we provide regional as well as country-level reports. Other than this we also provide a sectional report. Please get in contact with our sales representatives.
Press Release

Global Pharmaceutical Manufacturing Market to Reach $1.38 Trillion by 2035 with 7.35% CAGR, New Research Shows

The Global Mammography Market Is Estimated To Record a CAGR of Around 10.29% During The Forecast Period

Glue Stick Market to Reach USD 2.35 Billion by 2034

Podiatry Service Market to Reach USD 11.88 Billion by 2034

Microfluidics Technology Market to Reach USD 32.58 Billion by 2034

Ferric Chloride Market to Reach USD 10.65 Billion by 2034

Family Practice EMR Software Market to Reach USD 21.52 Billion by 2034

Electric Hairbrush Market to Reach USD 15.95 Billion by 2034

Daily Bamboo Products Market to Reach USD 143.52 Billion by 2034

Cross-border E-commerce Logistics Market to Reach USD 112.65 Billion by 2034
Table of Content
Chapter 1. Executive Summary Chapter 2. Scope Of The Study 2.1. Market Definition 2.2. Scope Of The Study 2.2.1. Objectives of Report 2.2.2. Limitations 2.3. Market Structure Chapter 3. Evolve BI Methodology Chapter 4. Market Insights and Trends 4.1. Supply/ Value Chain Analysis 4.1.1. Raw End Users Providers 4.1.2. Manufacturing Process 4.1.3. Distributors/Retailers 4.1.4. End-Use Industry 4.2. Porter’s Five Forces Analysis 4.2.1. Threat Of New Entrants 4.2.2. Bargaining Power Of Buyers 4.2.3. Bargaining Power Of Suppliers 4.2.4. Threat Of Substitutes 4.2.5. Industry Rivalry 4.3. Impact Of COVID-19 on the Epilepsy Devices Market 4.3.1. Impact on Market Size 4.3.2. End-Use Industry Trend, Preferences, and Budget Impact 4.3.3. Regulatory Framework/Government Policies 4.3.4. Key Players' Strategy to Tackle Negative Impact 4.3.5. Opportunity Window 4.4. Technology Overview 12.28. Macro factor 4.6. Micro Factor 4.7. Demand Supply Gap Analysis of the Epilepsy Devices Market 4.8. Import Analysis of the Epilepsy Devices Market 4.9. Export Analysis of the Epilepsy Devices Market Chapter 5. Market Dynamics 5.1. Introduction 5.2. DROC Analysis 5.2.1. Drivers 5.2.2. Restraints 5.2.3. Opportunities 5.2.4. Challenges 5.3. Patent Analysis 5.4. Industry Roadmap 5.5. Parent/Peer Market Analysis Chapter 6. Global Epilepsy Devices Market, By Product Type 6.1. Introduction 6.2. Conventional Devices 6.3. Wearable Devices 6.4. Others Chapter 7. Global Epilepsy Devices Market, By End User 7.1. Introduction 7.2. Hospitals & Clinics 7.3. Home Care Settings 7.4. Others Chapter 8. Global Epilepsy Devices Market, By Region 8.1. Introduction 8.2. North America 8.2.1. Introduction 8.2.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.2.3. Market Size and Forecast, By Country, 2023-2033 8.2.4. Market Size and Forecast, By Product Type, 2023-2033 8.2.5. Market Size and Forecast, By End User, 2023-2033 8.2.6. US 8.2.6.1. Introduction 8.2.6.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.2.6.3. Market Size and Forecast, By Product Type, 2023-2033 8.2.6.4. Market Size and Forecast, By End User, 2023-2033 8.2.7. Canada 8.2.7.1. Introduction 8.2.7.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.2.7.4. Market Size and Forecast, By Product Type, 2023-2033 8.2.7.5. Market Size and Forecast, By End User, 2023-2033 8.3. Europe 8.3.1. Introduction 8.3.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.3. Market Size and Forecast, By Country, 2023-2033 8.3.4. Market Size and Forecast, By Product Type, 2023-2033 8.3.5. Market Size and Forecast, By End User, 2023-2033 8.3.6. Germany 8.3.6.1. Introduction 8.3.6.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.6.3. Market Size and Forecast, By Product Type, 2023-2033 8.3.6.4. Market Size and Forecast, By End User, 2023-2033 8.3.7. France 8.3.7.1. Introduction 8.3.7.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.7.3. Market Size and Forecast, By Product Type, 2023-2033 8.3.7.4. Market Size and Forecast, By End User, 2023-2033 8.3.8. UK 8.3.8.1. Introduction 8.3.8.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.8.3. Market Size and Forecast, By Product Type, 2023-2033 8.3.8.4. Market Size and Forecast, By End User, 2023-2033 8.3.9. Italy 8.3.9.1. Introduction 8.3.9.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.9.3. Market Size and Forecast, By Product Type, 2023-2033 8.3.9.4. Market Size and Forecast, By End User, 2023-2033 8.3.11. Rest Of Europe 8.3.11.1. Introduction 8.3.11.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.11.3. Market Size and Forecast, By Product Type, 2023-2033 8.3.11.4. Market Size and Forecast, By End User, 2023-2033 8.4. Asia-Pacific 8.4.1. Introduction 8.4.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.3. Market Size and Forecast, By Country, 2023-2033 8.4.4. Market Size and Forecast, By Product Type, 2023-2033 8.12.28. Market Size and Forecast, By End User, 2023-2033 8.4.6. China 8.4.6.1. Introduction 8.4.6.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.6.3. Market Size and Forecast, By Product Type, 2023-2033 8.4.6.4. Market Size and Forecast, By End User, 2023-2033 8.4.7. India 8.4.7.1. Introduction 8.4.7.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.7.3. Market Size and Forecast, By Product Type, 2023-2033 8.4.7.4. Market Size and Forecast, By End User, 2023-2033 8.4.8. Japan 8.4.8.1. Introduction 8.4.8.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.8.3. Market Size and Forecast, By Product Type, 2023-2033 8.4.8.4. Market Size and Forecast, By End User, 2023-2033 8.4.9. South Korea 8.4.9.1. Introduction 8.4.9.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.9.3. Market Size and Forecast, By Product Type, 2023-2033 8.4.9.4. Market Size and Forecast, By End User, 2023-2033 8.4.10. Rest Of Asia-Pacific 8.4.10.1. Introduction 8.4.10.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.10.3. Market Size and Forecast, By Product Type, 2023-2033 8.4.10.4. Market Size and Forecast, By End User, 2023-2033 8.5. Rest Of The World (RoW) 8.5.1. Introduction 8.5.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.5.3. Market Size and Forecast, By Product Type, 2023-2033 8.5.4. Market Size and Forecast, By End User, 2023-2033 Chapter 9. Company Landscape 9.1. Introduction 9.2. Vendor Share Analysis 9.3. Key Development Analysis 9.4. Competitor Dashboard Chapter 10. Company Profiles 10.1. Natus Medical 10.1.1. Business Overview 10.1.2. Government & Defense Analysis 10.1.2.1. Government & Defense – Existing/Funding 10.1.3. Product Portfolio 10.1.4. Recent Development and Strategies Adopted 10.1.5. SWOT Analysis 10.2. Koninklijke Philips 10.2.1. Business Overview 10.2.2. Government & Defense Analysis 10.2.2.1. Government & Defense – Existing/Funding 10.2.3. Product Portfolio 10.2.4. Recent Development and Strategies Adopted 10.2.5. SWOT Analysis 10.3. Medtronic 10.3.1. Business Overview 10.3.2. Government & Defense Analysis 10.3.2.1. Government & Defense – Existing/Funding 10.3.3. Product Portfolio 10.3.4. Recent Development and Strategies Adopted 10.3.5. SWOT Analysis 10.4. Nihon Kohden 10.4.1. Business Overview 10.4.2. Government & Defense Analysis 10.4.2.1. Government & Defense – Existing/Funding 10.4.3. Product Portfolio 10.4.4. Recent Development and Strategies Adopted 10.12.28. SWOT Analysis 10.5. PulseGuard International Ltd. 10.5.1. Business Overview 10.5.2. Government & Defense Analysis 10.5.2.1. Government & Defense – Existing/Funding 10.5.3. Product Portfolio 10.5.4. Recent Development and Strategies Adopted 10.5.5. SWOT Analysis 10.6. Masimo Corporation 10.6.1. Business Overview 10.6.2. Government & Defense Analysis 10.6.2.1. Government & Defense – Existing/Funding 10.6.3. Product Portfolio 10.6.4. Recent Development and Strategies Adopted 10.6.5. SWOT Analysis 10.7. Empatica, Inc 10.7.1. Business Overview 10.7.2. Government & Defense Analysis 10.7.2.1. Government & Defense – Existing/Funding 10.7.3. Product Portfolio 10.7.4. Recent Development and Strategies Adopted 10.7.5. SWOT Analysis 10.8 Abbott Laboratories 10.8.1. Business Overview 10.8.2. Government & Defense Analysis 10.8.2.1. Government & Defense – Existing/Funding 10.8.3. Product Portfolio 10.8.4. Recent Development and Strategies Adopted 10.8.5. SWOT Analysis 10.9 LivaNova PLC 10.9.1. Business Overview 10.9.2. Government & Defense Analysis 10.9.2.1. Government & Defense – Existing/Funding 10.9.3. Product Portfolio 10.9.4. Recent Development and Strategies Adopted 10.9.5. SWOT Analysis 10.10. COMPUMEDICS 10.10.1. Business Overview 10.10.2. Government & Defense Analysis 10.10.2.1. Government & Defense – Existing/Funding 10.10.3. Product Portfolio 10.10.4. Recent Development and Strategies Adopted 10.10.5. SWOT Analysis
Connect to Analyst
Research Methodology


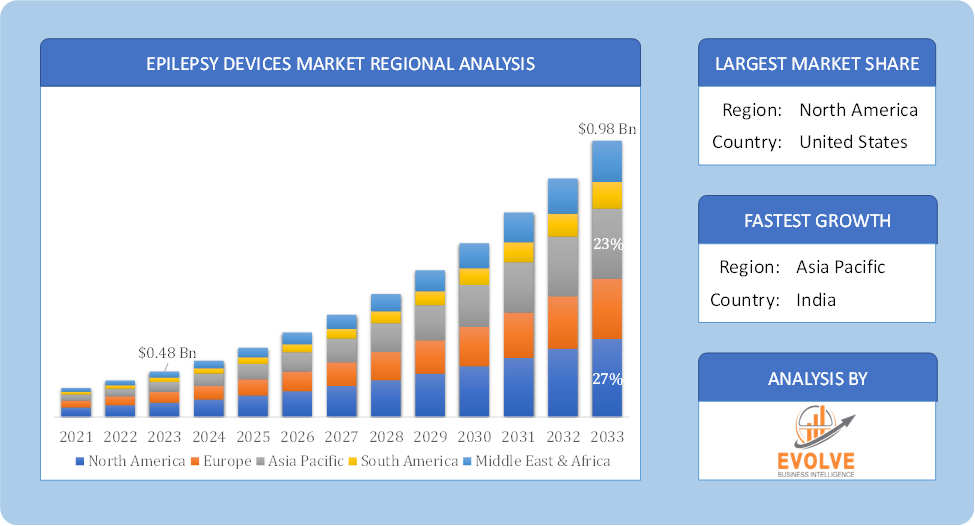 Epilepsy Devices North America Market
Epilepsy Devices North America Market

