Clinical Trial Imaging Services Market Analysis and Global Forecast 2023-2033
$ 1,390.00 – $ 5,520.00Price range: $ 1,390.00 through $ 5,520.00
Clinical Trial Imaging Services Market Research Report: Information By Modality (Computed Tomography, Magnetic Resonance Imaging, Positron Emission Tomography, Ultrasound, Others), By Application (Pharmaceutical Companies, Biotechnology Companies, Medical Device Manufacturers, Contract Research Organizations, Others), and by Region — Forecast till 2033
Page: 171
Clinical Trial Imaging Services Market Overview
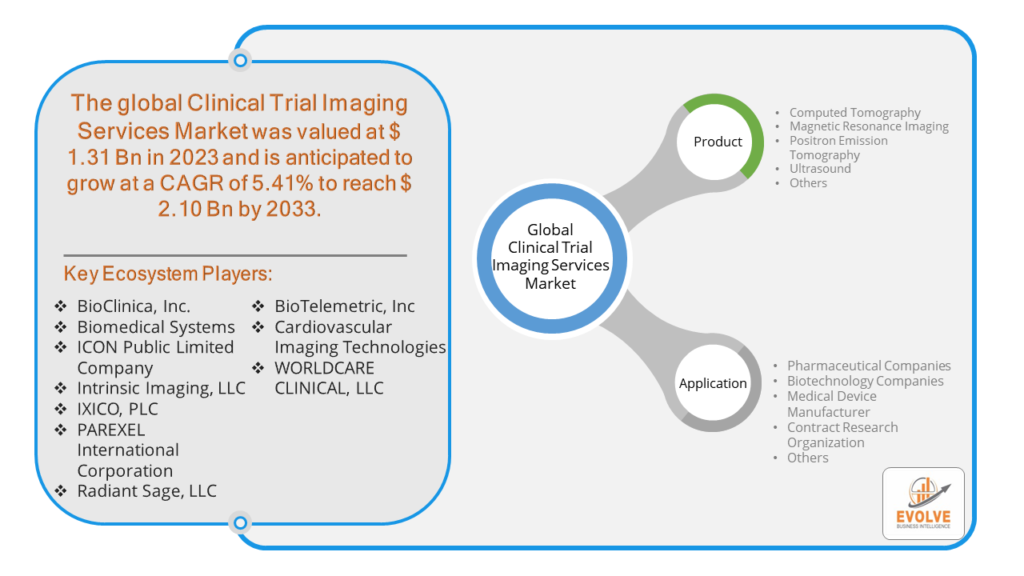
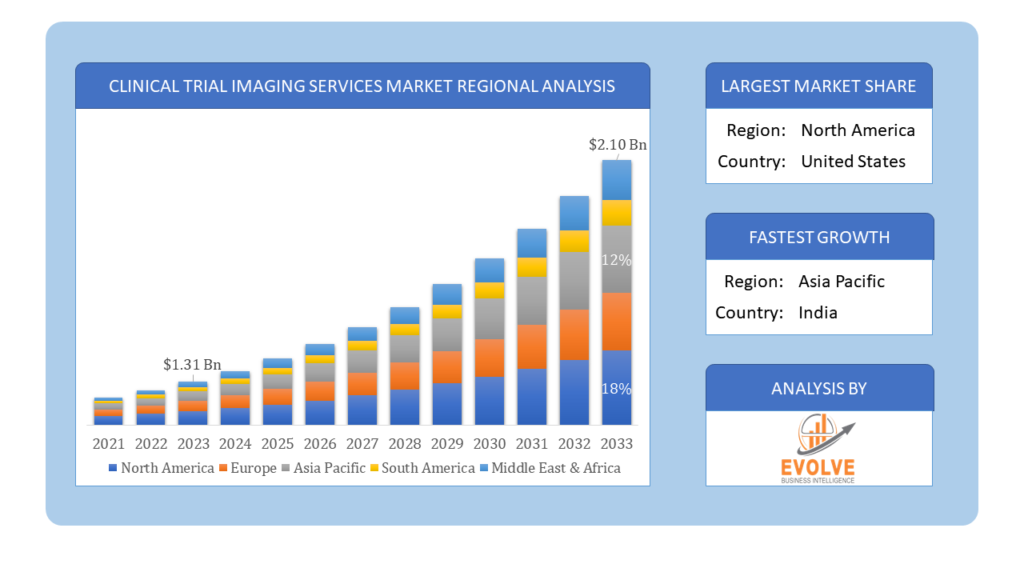
The Clinical Trial Imaging Services Market Size is expected to reach USD 2.10 Billion by 2033. The Clinical Trial Imaging Services industry size accounted for USD 1.31 Billion in 2023 and is expected to expand at a compound annual growth rate (CAGR) of 5.41% from 2023 to 2033. The Clinical Trial Imaging Services Market involves the provision of specialized imaging services to support clinical trials, particularly in the pharmaceutical and biotechnology industries. These services include image acquisition, analysis, and management, utilizing advanced imaging modalities like MRI, CT, PET, and ultrasound. They play a crucial role in assessing the efficacy and safety of new therapies, enhancing the precision of trial outcomes. The market is driven by the growing need for high-quality, standardized imaging data, advancements in imaging technologies, and increasing clinical trial activities globally.
Global Clinical Trial Imaging Services Market Synopsis
The COVID-19 pandemic has led to supply chain disruptions leading to supply shortages or lower demand in the clinical trial imaging services market. The travel restrictions and social-distancing measures have resulted in a sharp drop in consumer and business spending and this pattern is to continue for some time. The end-user trend and preferences have changed due to the pandemic and have resulted in manufacturers, developers, and service providers to adopt various strategies to stabilize the company.
Clinical Trial Imaging Services Market Dynamics
The major factors that have impacted the growth of Clinical Trial Imaging Services are as follows:
Drivers:
Ø Technological Advancements
Innovations in imaging technology, such as high-resolution MRI, CT, PET, and ultrasound, have significantly enhanced the ability to capture and analyze detailed images. These advancements improve the accuracy of diagnostics and monitoring in clinical trials, leading to better data quality and more reliable results.
Restraint:
- Data Management and Integration Issues
Managing and integrating large volumes of imaging data generated during clinical trials is a complex task. Ensuring data consistency, security, and accessibility while integrating imaging data with other clinical trial data poses significant technical and logistical challenges. Inadequate data management solutions can compromise the quality and reliability of trial outcomes.
Opportunity:
⮚ Development of Hybrid Imaging Technologies
The development and adoption of hybrid imaging technologies, such as PET/CT and PET/MRI, offer comprehensive insights by combining anatomical and functional imaging. These advanced modalities can provide more accurate and detailed information about disease states and treatment effects, enhancing the overall quality of clinical trial data.
Clinical Trial Imaging Services Segment Overview
By Modality
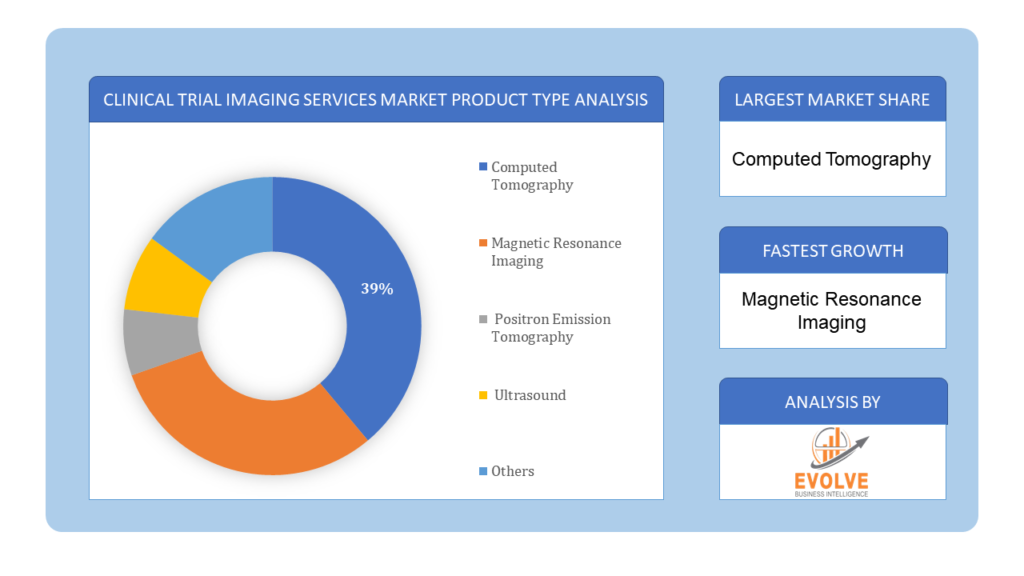 Based on Modality, the market is segmented based on Computed Tomography, Magnetic Resonance Imaging, Positron Emission Tomography, Ultrasound, Others. Positron Emission Tomography (PET) dominating the market due to their widespread use in clinical trials for assessing treatment efficacy and safety
Based on Modality, the market is segmented based on Computed Tomography, Magnetic Resonance Imaging, Positron Emission Tomography, Ultrasound, Others. Positron Emission Tomography (PET) dominating the market due to their widespread use in clinical trials for assessing treatment efficacy and safety
By Application
Based on Applications, the market has been divided into the Pharmaceutical Companies, Biotechnology Companies, Medical Device Manufacturers, Contract Research Organizations, Others. The clinical trial imaging services market is segmented based on end users, with pharmaceutical companies dominating the market due to the significant demand for advanced imaging technologies in pharmaceutical research and development
Global Clinical Trial Imaging Services Market Regional Analysis
Based on region, the global Clinical Trial Imaging Services market has been divided into North America, Europe, Asia-Pacific, the Middle East & Africa, and Latin America. North America is projected to dominate the use of the Clinical Trial Imaging Services market followed by the Asia-Pacific and Europe regions.
 Clinical Trial Imaging Services North America Market
Clinical Trial Imaging Services North America Market
North America holds a dominant position in the Clinical Trial Imaging Services Market. In North America, the clinical trial imaging services market is characterized by robust infrastructure, advanced healthcare facilities, and stringent regulatory frameworks supporting clinical trials. The region hosts numerous pharmaceutical and biotechnology companies conducting extensive research and development activities, driving demand for imaging services. Key factors contributing to market growth include the increasing prevalence of chronic diseases, rising investments in healthcare research, and the adoption of innovative imaging technologies. Moreover, collaborations between academic institutions, research organizations, and imaging service providers further enhance capabilities in conducting efficient and compliant clinical trials.
Clinical Trial Imaging Services Asia-Pacific Market
In the Asia Pacific region, the clinical trial imaging services market is experiencing rapid growth driven by increasing clinical research activities, rising healthcare investments, and expanding pharmaceutical and biotechnology sectors. Countries like China, India, Japan, and South Korea are key contributors to market expansion due to their large patient populations, improving healthcare infrastructure, and government initiatives to promote clinical trials. Key factors driving market growth include the region’s cost-effective clinical trial services, advancements in medical imaging technologies, and a growing emphasis on precision medicine and personalized healthcare.
Competitive Landscape
The global Clinical Trial Imaging Services market is highly competitive, with numerous players offering a wide range of software solutions. The competitive landscape is characterized by the presence of established companies, as well as emerging startups and niche players. To increase their market position and attract a wide consumer base, the businesses are employing various strategies, such as product launches, and strategic alliances.
Prominent Players:
- BioClinica, Inc.
- Biomedical Systems
- ICON Public Limited Company
- Intrinsic Imaging, LLC
- IXICO, PLC
- PAREXEL International Corporation
- Radiant Sage, LLC
- BioTelemetric, Inc
- Cardiovascular Imaging Technologies
- WORLDCARE CLINICAL, LLC
Key Development
In September 2022, Parexel International was recognized by Frost & Sullivan with the 2022 Global Customer Value Leadership Award for its excellence in planning, operationalizing, and delivering decentralized clinical trials (DCTs) worldwide via leading-edge technology.
Scope of the Report
Global Clinical Trial Imaging Services Market, by Product
- Computed Tomography
- Magnetic Resonance Imaging
- Positron Emission Tomography
- Ultrasound
- Others
Global Clinical Trial Imaging Services Market, by Application
- Pharmaceutical Companies
- Biotechnology Companies
- Medical Device Manufacturer
- Contract Research Organization
- Others
Global Clinical Trial Imaging Services Market, by Region
- North America
- US
- Canada
- Mexico
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Benelux
- Nordic
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- Indonesia
- Austalia
- Malaysia
- India
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- South Africa
- Rest of Middle East & Africa
| Parameters | Indicators |
|---|---|
| Market Size | 2033: $2.10 Billion |
| CAGR | 5.41% CAGR (2023-2033) |
| Base year | 2022 |
| Forecast Period | 2023-2033 |
| Historical Data | 2021 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, and Trends |
| Key Segmentations | Modality, Application |
| Geographies Covered | North America, Europe, Asia-Pacific, Latin America, Middle East, Africa |
| Key Vendors | BioClinica, Inc., Biomedical Systems, ICON Public Limited Company, Intrinsic Imaging, LLC, IXICO, PLC, PAREXEL International Corporation, Radiant Sage, LLC, BioTelemetric, Inc, Cardiovascular Imaging Technologies, WORLDCARE CLINICAL, LLC |
| Key Market Opportunities | • The rise of e-commerce |
| Key Market Drivers | • Technological Advancements • Increasing Urbanization |
REPORT CONTENT BRIEF:
- High-level analysis of the current and future Clinical Trial Imaging Services market trends and opportunities
- Detailed analysis of current market drivers, restraining factors, and opportunities in the future
- Clinical Trial Imaging Services market historical market size for the year 2021, and forecast from 2023 to 2033
- Clinical Trial Imaging Services market share analysis at each product level
- Competitor analysis with detailed insight into its product segment, Government & Defense strength, and strategies adopted.
- Identifies key strategies adopted including product launches and developments, mergers and acquisitions, joint ventures, collaborations, and partnerships as well as funding taken and investment done, among others.
- To identify and understand the various factors involved in the global Clinical Trial Imaging Services market affected by the pandemic
- To provide a detailed insight into the major companies operating in the market. The profiling will include the Government & Defense health of the company’s past 2-3 years with segmental and regional revenue breakup, product offering, recent developments, SWOT analysis, and key strategies.
Press Release

Global Pharmaceutical Manufacturing Market to Reach $1.38 Trillion by 2035 with 7.35% CAGR, New Research Shows

The Global Mammography Market Is Estimated To Record a CAGR of Around 10.29% During The Forecast Period

Glue Stick Market to Reach USD 2.35 Billion by 2034

Podiatry Service Market to Reach USD 11.88 Billion by 2034

Microfluidics Technology Market to Reach USD 32.58 Billion by 2034

Ferric Chloride Market to Reach USD 10.65 Billion by 2034

Family Practice EMR Software Market to Reach USD 21.52 Billion by 2034

Electric Hairbrush Market to Reach USD 15.95 Billion by 2034

Daily Bamboo Products Market to Reach USD 143.52 Billion by 2034

Cross-border E-commerce Logistics Market to Reach USD 112.65 Billion by 2034
Frequently Asked Questions (FAQ)
What is the study period of the Clinical Trial Imaging Services Market?
The study period for the Clinical Trial Imaging Services Market is from 2023 to 2033.
What is the growth rate of the Clinical Trial Imaging Services Market?
The Clinical Trial Imaging Services Market is expected to expand at a compound annual growth rate (CAGR) of 5.41% from 2023 to 2033.
Which region has the highest growth rate in the Clinical Trial Imaging Services Market?
The Asia-Pacific region is experiencing rapid growth in the Clinical Trial Imaging Services Market due to increasing clinical research activities and healthcare investments.
Which region has the largest share of the Clinical Trial Imaging Services Market?
North America holds the dominant position in the global Clinical Trial Imaging Services Market.
Who are the key players in the Clinical Trial Imaging Services Market?
Key players in the Clinical Trial Imaging Services Market include BioClinica, Inc., Biomedical Systems, ICON Public Limited Company, Intrinsic Imaging, LLC, IXICO, PLC, PAREXEL International Corporation, Radiant Sage, LLC, BioTelemetric, Inc., Cardiovascular Imaging Technologies, and WORLDCARE CLINICAL, LLC.
Do you offer Post sales support?
Yes, we offer 16 hours of analyst support to solve the queries
Do you sell particular sections of a report?
Yes, we provide regional as well as country-level reports. Other than this we also provide a sectional report. Please get in contact with our sales representatives.
Table of Content
Chapter 1. Executive Summary Chapter 2. Scope Of The Study 2.1. Market Definition 2.2. Scope Of The Study 2.2.1. Objectives of Report 2.2.2. Limitations 2.3. Market Structure Chapter 3. Evolve BI Methodology Chapter 4. Market Insights and Trends 4.1. Supply/ Value Chain Analysis 4.1.1. Raw Applications Providers 4.1.2. Manufacturing Process 4.1.3. Distributors/Retailers 4.1.4. End-Use Industry 4.2. Porter’s Five Forces Analysis 4.2.1. Threat Of New Entrants 4.2.2. Bargaining Power Of Buyers 4.2.3. Bargaining Power Of Suppliers 4.2.4. Threat Of Substitutes 4.2.5. Industry Rivalry 4.3. Impact Of COVID-19 on the Clinical Trial Imaging Services Market 4.3.1. Impact on Market Size 4.3.2. End-Use Industry Trend, Preferences, and Budget Impact 4.3.3. Regulatory Framework/Government Policies 4.3.4. Key Players' Strategy to Tackle Negative Impact 4.3.5. Opportunity Window 4.4. Technology Overview 12.28. Macro factor 4.6. Micro Factor 4.7. Demand Supply Gap Analysis of the Clinical Trial Imaging Services Market 4.8. Import Analysis of the Clinical Trial Imaging Services Market 4.9. Export Analysis of the Clinical Trial Imaging Services Market Chapter 5. Market Dynamics 5.1. Introduction 5.2. DROC Analysis 5.2.1. Drivers 5.2.2. Restraints 5.2.3. Opportunities 5.2.4. Challenges 5.3. Patent Analysis 5.4. Industry Roadmap 5.5. Parent/Peer Market Analysis Chapter 6. Global Clinical Trial Imaging Services Market, By Modality 6.1. Introduction 6.2. Computed Tomography 6.3. Magnetic Resonance Imaging 6.4. Positron Emission Tomography 6.5. Ultrasound 6.6. Others Chapter 7. Global Clinical Trial Imaging Services Market, By Application 7.1. Introduction 7.2. Pharmaceutical Companies 7.3. Biotechnology Companies 7.4. Medical Device Manufacturer 7.5. Contract Research Organization 7.6. Others. Chapter 8. Global Clinical Trial Imaging Services Market, By Region 8.1. Introduction 8.2. North America 8.2.1. Introduction 8.2.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.2.3. Market Size and Forecast, By Country, 2023-2033 8.2.4. Market Size and Forecast, By Modality, 2023-2033 8.2.5. Market Size and Forecast, By Application, 2023-2033 8.2.6. US 8.2.6.1. Introduction 8.2.6.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.2.6.3. Market Size and Forecast, By Modality, 2023-2033 8.2.6.4. Market Size and Forecast, By Application, 2023-2033 8.2.7. Canada 8.2.7.1. Introduction 8.2.7.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.2.7.4. Market Size and Forecast, By Modality, 2023-2033 8.2.7.5. Market Size and Forecast, By Application, 2023-2033 8.3. Europe 8.3.1. Introduction 8.3.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.3. Market Size and Forecast, By Country, 2023-2033 8.3.4. Market Size and Forecast, By Modality, 2023-2033 8.3.5. Market Size and Forecast, By Application, 2023-2033 8.3.6. Germany 8.3.6.1. Introduction 8.3.6.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.6.3. Market Size and Forecast, By Modality, 2023-2033 8.3.6.4. Market Size and Forecast, By Application, 2023-2033 8.3.7. France 8.3.7.1. Introduction 8.3.7.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.7.3. Market Size and Forecast, By Modality, 2023-2033 8.3.7.4. Market Size and Forecast, By Application, 2023-2033 8.3.8. UK 8.3.8.1. Introduction 8.3.8.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.8.3. Market Size and Forecast, By Modality, 2023-2033 8.3.8.4. Market Size and Forecast, By Application, 2023-2033 8.3.9. Italy 8.3.9.1. Introduction 8.3.9.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.9.3. Market Size and Forecast, By Modality, 2023-2033 8.3.9.4. Market Size and Forecast, By Application, 2023-2033 8.3.11. Rest Of Europe 8.3.11.1. Introduction 8.3.11.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.3.11.3. Market Size and Forecast, By Modality, 2023-2033 8.3.11.4. Market Size and Forecast, By Application, 2023-2033 8.4. Asia-Pacific 8.4.1. Introduction 8.4.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.3. Market Size and Forecast, By Country, 2023-2033 8.4.4. Market Size and Forecast, By Modality, 2023-2033 8.12.28. Market Size and Forecast, By Application, 2023-2033 8.4.6. China 8.4.6.1. Introduction 8.4.6.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.6.3. Market Size and Forecast, By Modality, 2023-2033 8.4.6.4. Market Size and Forecast, By Application, 2023-2033 8.4.7. India 8.4.7.1. Introduction 8.4.7.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.7.3. Market Size and Forecast, By Modality, 2023-2033 8.4.7.4. Market Size and Forecast, By Application, 2023-2033 8.4.8. Japan 8.4.8.1. Introduction 8.4.8.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.8.3. Market Size and Forecast, By Modality, 2023-2033 8.4.8.4. Market Size and Forecast, By Application, 2023-2033 8.4.9. South Korea 8.4.9.1. Introduction 8.4.9.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.9.3. Market Size and Forecast, By Modality, 2023-2033 8.4.9.4. Market Size and Forecast, By Application, 2023-2033 8.4.10. Rest Of Asia-Pacific 8.4.10.1. Introduction 8.4.10.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.4.10.3. Market Size and Forecast, By Modality, 2023-2033 8.4.10.4. Market Size and Forecast, By Application, 2023-2033 8.5. Rest Of The World (RoW) 8.5.1. Introduction 8.5.2. Driving Factors, Opportunity Analyzed, and Key Trends 8.5.3. Market Size and Forecast, By Modality, 2023-2033 8.5.4. Market Size and Forecast, By Application, 2023-2033 Chapter 9. Company Landscape 9.1. Introduction 9.2. Vendor Share Analysis 9.3. Key Development Analysis 9.4. Competitor Dashboard Chapter 10. Company Profiles 10.1. BioClinica, Inc. 10.1.1. Business Overview 10.1.2. Government & Defense Analysis 10.1.2.1. Government & Defense – Existing/Funding 10.1.3. Product Portfolio 10.1.4. Recent Development and Strategies Adopted 10.1.5. SWOT Analysis 10.2. Biomedical Systems 10.2.1. Business Overview 10.2.2. Government & Defense Analysis 10.2.2.1. Government & Defense – Existing/Funding 10.2.3. Product Portfolio 10.2.4. Recent Development and Strategies Adopted 10.2.5. SWOT Analysis 10.3. ICON Public Limited Company 10.3.1. Business Overview 10.3.2. Government & Defense Analysis 10.3.2.1. Government & Defense – Existing/Funding 10.3.3. Product Portfolio 10.3.4. Recent Development and Strategies Adopted 10.3.5. SWOT Analysis 10.4. Intrinsic Imaging, LLC 10.4.1. Business Overview 10.4.2. Government & Defense Analysis 10.4.2.1. Government & Defense – Existing/Funding 10.4.3. Product Portfolio 10.4.4. Recent Development and Strategies Adopted 10.12.28. SWOT Analysis 10.5. IXICO, PLC 10.5.1. Business Overview 10.5.2. Government & Defense Analysis 10.5.2.1. Government & Defense – Existing/Funding 10.5.3. Product Portfolio 10.5.4. Recent Development and Strategies Adopted 10.5.5. SWOT Analysis 10.6. PAREXEL International Corporation 10.6.1. Business Overview 10.6.2. Government & Defense Analysis 10.6.2.1. Government & Defense – Existing/Funding 10.6.3. Product Portfolio 10.6.4. Recent Development and Strategies Adopted 10.6.5. SWOT Analysis 10.7. Radiant Sage, LLC 10.7.1. Business Overview 10.7.2. Government & Defense Analysis 10.7.2.1. Government & Defense – Existing/Funding 10.7.3. Product Portfolio 10.7.4. Recent Development and Strategies Adopted 10.7.5. SWOT Analysis 10.8 BioTelemetric, Inc 10.8.1. Business Overview 10.8.2. Government & Defense Analysis 10.8.2.1. Government & Defense – Existing/Funding 10.8.3. Product Portfolio 10.8.4. Recent Development and Strategies Adopted 10.8.5. SWOT Analysis 10.9 Cardiovascular Imaging Technologies 10.9.1. Business Overview 10.9.2. Government & Defense Analysis 10.9.2.1. Government & Defense – Existing/Funding 10.9.3. Product Portfolio 10.9.4. Recent Development and Strategies Adopted 10.9.5. SWOT Analysis 10.10. WORLDCARE CLINICAL, LLC 10.10.1. Business Overview 10.10.2. Government & Defense Analysis 10.10.2.1. Government & Defense – Existing/Funding 10.10.3. Product Portfolio 10.10.4. Recent Development and Strategies Adopted 10.10.5. SWOT Analysis
Connect to Analyst
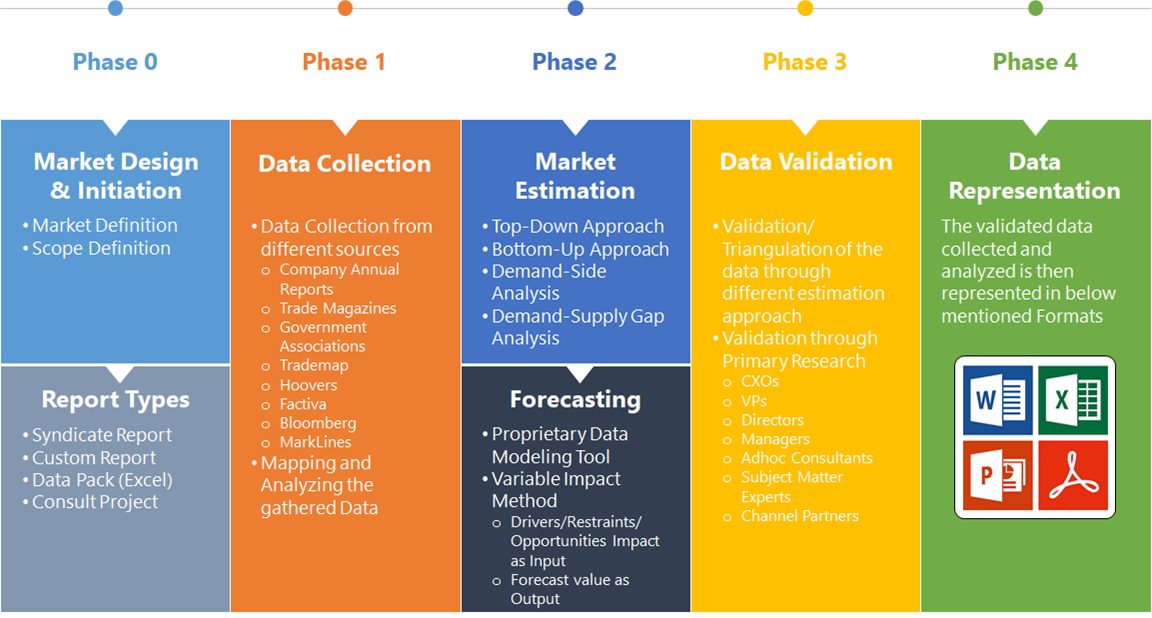
Research Methodology