Pharmaceutical Quality Control Market Overview
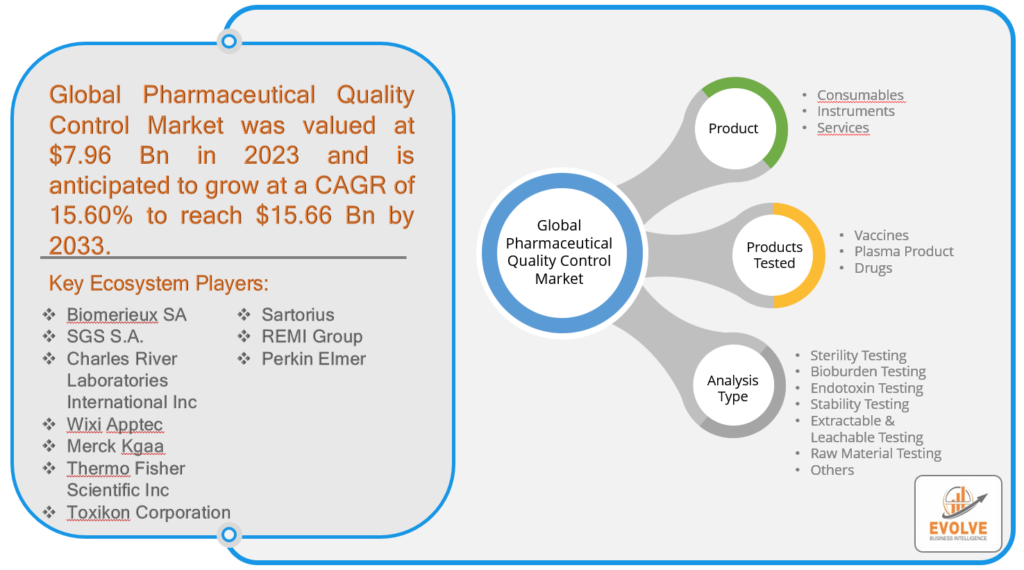
Pharmaceutical Quality Control Market Size is expected to reach USD 15.66 Billion by 2033. ThePharmaceutical Quality Controlindustry size accounted for USD 7.96 Billion in 2023 and is expected to expand at a compound annual growth rate (CAGR) of 15.60% from 2023 to 2033.Pharmaceutical Quality Control is a process of verifying that pharmaceutical products conform to the specified quality standards in terms of safety, efficacy, and purity. This involves a comprehensive series of tests, inspections, and evaluations at different stages of the manufacturing process to detect any deviations from the expected quality parameters. The QC activities commence with the testing of raw materials and continue throughout the manufacturing process, including in-process and finished product testing, and stability testing to ascertain the product’s long-term stability. The tests performed during QC may comprise physical, chemical, and microbiological analyses, in addition to the evaluation of product performance and packaging. The primary objective of pharmaceutical QC is to ensure that the products are consistent in terms of quality, potency, and purity, and comply with regulatory requirements and customer expectations. This is a crucial element of pharmaceutical manufacturing that serves to prevent defects, maintain product quality, and guarantee patient safety.
The COVID-19 pandemic has certainly had a significant impact on the pharmaceutical industry, including the pharmaceutical quality control market. The pandemic has led to a surge in demand for pharmaceutical products, including vaccines, therapeutics, and diagnostics, which has increased the need for stringent quality control measures. With the urgent need for COVID-19 treatments and vaccines, regulatory agencies such as the FDA and EMA have expedited the approval process, but have also placed a heightened emphasis on ensuring that these products are safe and effective. As a result, pharmaceutical companies have had to invest more heavily in quality control measures to ensure that their products meet the required standards. Moreover, the pandemic has highlighted the importance of global supply chain resilience and the need for robust quality control measures to ensure the safety and efficacy of pharmaceutical products. This has led to an increased focus on quality control across the entire pharmaceutical industry, including both small and large-scale manufacturers.
Global Pharmaceutical Quality Control Market Growth Factors
- Increasing focus on personalized medicine
The increasing prevalence of chronic diseases is one of the major drivers of the market. Chronic diseases, such as cancer, cardiovascular diseases, diabetes, and respiratory diseases, are significant public health challenges worldwide. According to the World Health Organization (WHO), chronic diseases are responsible for approximately 71% of all deaths globally. The rising incidence of chronic diseases has led to an increased demand for pharmaceutical products, including drugs and biologics. These products require rigorous quality control measures to ensure that they are safe and effective for patient use. Pharmaceutical quality control plays a critical role in ensuring that these products meet the required quality standards and are suitable for use in the treatment of chronic diseases. Additionally, as the demand for pharmaceutical products for chronic diseases increases, so does the need for consistent and reliable quality control measures. This is particularly important for the production of generic drugs, which are often used to treat chronic diseases. Generic drugs must meet the same quality standards as their branded counterparts, and pharmaceutical quality control helps to ensure that these standards are met.
Global Pharmaceutical Quality Control Market Restraining Factors
- High cost of quality control
Implementing and maintaining quality control measures can be costly, particularly for small and mid-sized pharmaceutical companies. These costs can include expenses for specialized equipment, trained personnel, and compliance with regulatory requirements. The high cost of quality control can also impact drug pricing. Pharmaceutical companies may pass on the cost of quality control to consumers, resulting in higher drug prices. This may make pharmaceutical products less accessible to some patient populations, particularly those in developing countries or with limited access to healthcare. Moreover, the cost of quality control may also impact research and development (R&D) efforts. Pharmaceutical companies may be hesitant to invest in R&D for new drugs or biologics if the cost of quality control is too high, particularly for smaller companies with limited financial resources.
Global Pharmaceutical Quality Control Market Opportunity Analysis
advancements in technology are creating significant opportunities for the pharmaceutical quality control market. Automation, artificial intelligence, real-time monitoring, advanced analytics, and digitalization are transforming the pharmaceutical industry, and companies that can leverage these technologies to improve the accuracy, efficiency, and cost-effectiveness of quality control measures will be well-positioned to succeed in the market.
Global Pharmaceutical Quality Control Market Segment Analysis
The Consumables segment is expected to hold the largest market share in 2023
Based on the Product, the market is segmented based on Consumables, Instruments, and Services. The largest market share is anticipated to go to the Consumablessegment.Driven by elements including rising demand for analytical testing services and an increase in the quantity of product and medicine launches.
TheVaccinessegment is expected to hold the largest market share in 2023
Based on Products Tested, the market has been divided intoVaccines, Plasma Products, and Drugs.The Vaccinessegment is expected to hold the largest market share in the Market, due to increasing demand for preventive healthcare, government initiatives for vaccination programs, and the ongoing COVID-19 pandemic. The development and manufacturing of vaccines require stringent quality control measures to ensure their safety, efficacy, and potency.
The Sterility Testing segment is expected to hold the largest market share in 2023
Based on Analysis Type, the market has been divided into Sterility Testing, Bioburden Testing, Endotoxin Testing, Stability Testing, Extractable & Leachable Testing, Raw Material Testing, and Others.The market is projected to see significant growth in the sterility testing segment due to factors such as the rising demand for biologics and biosimilars, the expansion of the pharmaceutical and biotechnology industries, and the rise in hospital-acquired infections.
Global Pharmaceutical Quality Control Market, Segmental Chart
Based on region, the market has been divided into North America, Europe, Asia-Pacific, theMiddle East & Africa, and Latin America. The area of Europe is anticipated to dominate the market for the usage of Pharmaceutical Quality Control, followed by those in Asia-Pacific and North America.
Regional Coverage:
- North America
- US
- Canada
- Mexico
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Benelux
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- Indonesia
- Australia
- Malaysia
- Thailand
- India
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- South Africa
- Rest of Middle East & Africa
Europe Market
The Europe region is the leading market for Pharmaceutical Quality Control. Europe is home to several leading pharmaceutical companies, such as Roche, Novartis, and Sanofi, and has a robust regulatory framework for drug development and manufacturing. The European Medicines Agency (EMA) is the regulatory body responsible for approving and monitoring medicinal products in the European Union (EU) and plays a critical role in ensuring the quality, safety, and efficacy of medicines in the EU.
Asia Pacific Market
The pharmaceutical business in the Asia Pacific area has seen enormous growth as a result of several reasons, including a sizable population, rising demand for high-quality healthcare, and expanding pharmaceutical sector investments. There are numerous developing nations in the area, such as China, India, and Japan, who have made large investments in their healthcare industries.
Competitive Landscape
The competitive landscape includes key players (tier 1, tier 2, and local) having a presence across the globe. Companies such as Merck Kgaa, Thermo Fisher Scientific Inc, Toxikon Corporation, and Sartorius are some of the leading players in the global Pharmaceutical Quality ControlIndustry. These players have adopted partnership, acquisition, expansion, and new product development, among others as their key strategies.
Key Market Players:
- Biomerieux SA
- SGS S.A.
- Charles River Laboratories International Inc
- Wixi Apptec
- Merck Kgaa
- Thermo Fisher Scientific Inc
- Toxikon Corporation
- Sartorius
- REMI Group
- Perkin Elmer
Market Share Acquisition Strategies: Analysis of Key Approaches Employed by Top Players
On February2022, Merck announced the launch of a new quality control platform for Pharmaceutical Manufacturing.
On October 2021, Merck announced a collaboration with Vineti to Strengthen its Quality Control Capabilities.
Report Scope:
Global Pharmaceutical Quality Control Market, by Product
- Consumables
- Instruments
- Services
Global Pharmaceutical Quality Control Market, by Products Tested
- Vaccines
- Plasma Product
- Drugs
Global Pharmaceutical Quality Control Market, by Analysis Type
- Sterility Testing
- Bioburden Testing
- Endotoxin Testing
- Stability Testing
- Extractable & Leachable Testing
- Raw Material Testing
- Others
Pharmaceutical Quality Control Market Synopsis:
| PARAMETER | VALUE |
Market Size |
· 2023: USD 9.8 Billion· 2033: USD 15.66 Billion |
Growth Rate |
· First 5 Years CAGR (2023–2028): XX%· Last 5 Years CAGR (2028–2033): XX%· 10 Years CAGR (2023–2033): 15.60% |
Key Market Drivers |
· Increasing focus on personalized medicine· Increasing focus on patient safety |
Key Market Restraints |
· High cost of quality control |
Market Opportunities |
· Advancements in technology· Increasing regulatory focus on quality control |
Key Market Players |
· Biomerieux SA· SGS S.A.· Charles River Laboratories International Inc· Wixi Apptec· Merck Kgaa· Thermo Fisher Scientific Inc· Toxikon Corporation· Sartorius· REMI Group· Perkin Elmer |
REPORT CONTENT BRIEF:
- High-level analysis of the current and future Pharmaceutical Quality ControlIndustry trends and opportunities
- Detailed analysis of current market drivers, restraining factors, and opportunities analysis in the future
- Historical market size for the year 2021, and forecast from 2023 to 2033
- Pharmaceutical Quality Control market share analysis for each segment
- Competitor analysis with a comprehensive insight into its product segment, financial strength, and strategies adopted.
- Identifies key strategies adopted by the key players including new product development, mergers and acquisitions, joint ventures, collaborations, and partnerships.
- To identify and understand the various factors involved in the global Pharmaceutical Quality Control market affected by the pandemic
- To provide year-on-year growth from 2022 to 2033
- To provide short-term, long-term, and overall CAGR comparison from 2022 to 2033.
- Provide Total Addressable Market (TAM) for the Global Pharmaceutical Quality Control Market
Frequently Asked Questions (FAQ)
What are the 10 Years CAGR (2023 to 2033) of the global Pharmaceutical Quality Control market?
The global Pharmaceutical Quality Control market is growing at a CAGR of ~60% over the next 10 years
Which region has the highest growth rate in the market of Pharmaceutical Quality Control?
Asia Pacific is expected to register the highest CAGR during 2023-2033
Which region accounted for the largest share of the market of Pharmaceutical Quality Control?
Europe holds the largest share in 2022
Major Key Players in the Market of Pharmaceutical Quality Control?
Biomerieux, SGS S.A., Charles River Laboratories International Inc, Wixi Apptec, Merck Kgaa, Thermo Fisher Scientific Inc, Toxikon Corporation, Sartorius, REMI Group, Perkin Elmer are the major companies operating in the Pharmaceutical Quality Control
Do you offer Post Sale Support?
Yes, we offer 16 hours of analyst support to solve the queries
Do you deliver sections of a report?
Yes, we do provide regional as well as country-level reports. Other than this we also provide a sectional report. Please get in contact with our sales representatives.
